ORGANIC CHEMISTRY
advertisement

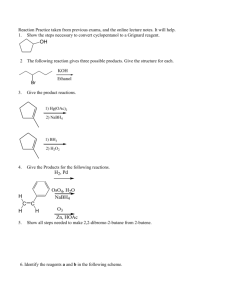
ORGANIC CHEMISTRY CH 399, Spring 2003 WORKSHOP 2 Carbonyl Compounds II 1. Give a reasonable mechanism for each of the following reactions. Use correct electron pushing. OCH3 N Ph dry HCl + CH3OH (excess) OCH3 + PhNH3 Cl O 1. Ph3P BrCH2CH2 CH2CH2 2. Me3COK 2. Provide structures or reagents for A through D. O A MeO H OMe H B cold dil. aq. KMnO4 C O D H NaBH 4 EtOH, H2O, OH C OH H 3C C H C H OH 3. Give structures for E and F and explain how they are consistent with the information given. Compound E (C7H16O2) was optically active, did not have IR absorption in the 1700 cm–1 region, and did not react with Tollen's reagent (Ag(NH3)2). When E was treated with dilute aqueous HCl, neutral compound F was obtained in addition to an equivalent each of methanol and ethanol. Compound F had IR absorption at 1730 cm–1 and reacted with Tollen's reagent to form a silver mirror. Upon acidification of the Tollen's reagent reaction, butanoic acid was isolated. 4. Consider and explain the following: If excess benzaldehyde is used in the Grignard reaction below, large amounts of benzophenone and benzyl alcohol are formed, in addition to the expected diphenylmethanol. Benzophenone and benzyl alcohol are formed in 1:1 ratio. i. Remembering that reducing agents react with oxidizing agents, analyze this reaction in redox terms. ii. Propose a mechanism for the formation of benzophenone and benzyl alcohol. O C H 1. PhMgBr 2. H3O O OH C H + + C CH2 OH excess 5. Propose methods for accomplishing the following conversions. Specify necessary reactants, reagents and conditions. O O2N C O a. C CH3 O Br b. alcohols with four or less carbons C H CH3 OH (CH3 )2CHCH CHCH(CH 3)2