Chemical Engineering E3830y
advertisement

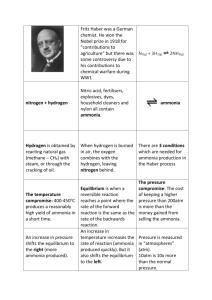
_________________________________________________ (your name, please print) April 29, 1998 Chemical Engineering E3830y Final Examination. Instructions (Please read carefully) Questions are organized according to the experiments they pertain to. Answer each question in the space provided immediately after the question. Put your name on each page. Examinations sheets will be separated for grading. You are allowed two hours. No books or notes are allowed. ABS 1. What factor was determined to characterize the ability of the column to remove ammonia from the incoming air stream? 2. Two methods were used to determine ammonia leaving in the exiting air stream. What were they? 3. Name one positive and one negative attribute of the type of packing used in this experiment? 4. Suppose that the packed column is tilted. How will this affect the packed bed efficiency in absorbing ammonia? 5. If you had to fill a very tall packed bed column with ceramic packing (that could break easily), how would you do it ? 6. How can you use a very simple and practical method to know (without the use of a thermometer or thermocouple) if ammonia adsorption in water is exothermic or endothermic? What is the answer? 7. Calculate the ammonia flow rate if you used 25ml 1N HCL and two drops of phenolphthalein in ethanol to adsorb pure ammonia gas and it took 85 seconds for the solution to turn pink. Page 1 of 5 _________________________________________________ (your name, please print) ELE 1. What are the reactions at the cathode and anode? 2. What happens to the current efficiency as the voltage increases to very high values? 3. By increasing flow rates, what do you expect in the transmittance of effluent? 4. How did you prevent bubbles from gathering at the top part of the cell? 5. What do you add to the effluent before you measure transmittances? Why? LIX 1. What are the three variables studied in the liquid-liquid extraction experiment? 2. How did you measure concentrations of copper in the effluent? 3. Why do you need to use a settler? 4. Explain how transmittance (T) and absorbance (A) are related each other. 5. Calculate a residence time for the CSTR when the flow rates of LIX and copper are 18ml/min and 20ml/min. Assume the volume of the CSTR is 260ml. Page 2 of 5 _________________________________________________ (your name, please print) OXY 1. Human blood has a capacity of 200 STP ml of blood per liter. Calculate the weight percent of sodium sulfite that is needed to match this capacity. Data: MW of sodium sulfite is 126; STP volume of 1 mole of a monovalent gas (such as He) is 22400 ml. 2. You were cautioned not to over-pressure either the blood side or the gas side of the oxygenator you studied. Answer these two questions: a) If the blood side were overpressured, what would be the observed effect on subsequent oxygen transport? Explain why (< 25 words). b) If the oxygen side were overpressured, what would be the observed effect on subsequent oxygen transport? Explain why (< 25 words). 3. How many ml of 0.01 M sodium thiosulfate are equivalent to 10 ml of sodium sulfite whose strength is 1 g/L? (Sulfite MW is 126.) 4. The membrane used in the oxygenator you studied was made of polyethylene, polypropylene, teflon, polysulfone (state one) and was a) hydrophilic or b) hydrophobic. (Two answers required.) Page 3 of 5 _________________________________________________ (your name, please print) ISO 1. If measurements of kinetics are made in the cis-Butene, trans-Butene, 1Butene system are made, and if the equilibrium constants are not known for the temperature used, how many independent kinetic equations can be formulated? 2. In the gas chromatograph used in this experiment identify: 1. The carrier gas 2. The nature of the column packing, 3. The kind of detector used to sense the hydrocarbon peaks. 3. If a reactor contains 5 g. of catalyst that has a pore volume of 0.29 ml/g catalyst, what is the mean residence time of the reactant stream in the pores when the flow of gas is 70 scc/min? Assume the reactor to be at 350 C. The bulk density of the catalyst is 2.9 gm/cc. 4. How can one tell whether ratios of product concentrations are equilibrium constants or not? 5. For the circumstances of this experiment, do you expect that the slope of a curve of ln (rate constant) vs. 1/T (necessarily taken from kinetic data) will be greater or less than the slope of a curve of ln (equilibrium constant) vs. 1/T (necessarily taken from equilibrium data)? In <25 words, justify your answer. Page 4 of 5 _________________________________________________ (your name, please print) HYD 1. In this experiment you measure the hydrolysis of ethyl acetate. The scenario proposes a reason for making these measurements. What is that reason? 2. Name the mechanistic steps that must be taken for hydrolysis to occur. Also name the independent variables that you changed during the experiment. 3. If one wishes to make this process maximally dependent on the kinetics of the hydrolysis how should he/she set (high vs. low) the independent variables? State each variable and whether it should be high or low. 4. Which of the following methods was used to follow the hydrolysis reaction: titration of acid formed; titration of base formed; change in product color using a spectrophotometer, liquid chromatography (HPLC), density changes. Page 5 of 5