midterm_solution
advertisement

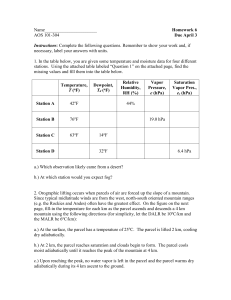
1. Consider two parcels of dry air that rise through the atmosphere from the surface (at 1000 hPa) to 800 hPa, maintaining mechanical equilibrium with the environment. Parcel A rises adiabatically, and Parcel B rises isothermally. (a) If the specific volume of both parcels at the surface is 0.8 m3, what is the specific volume of each parcel at 800 hPa? Comment on whether or not (and why) your calculation of the relative sizes of the parcels’ specific volumes makes physical sense to you. For the parcel rising adiabatically, one of Poisson’s equations can be used p s v s p800 v800 p v800 v s s p800 1 1000 0.714 0.8m 3 0.938m 3 800 For the parcel rising isothermally, the ideal gas law provides the constraint: v800 RT sfc p800 . The surface temperature is calculated using the ideal gas law applied at the surface, p s v s 0.8m 3 1000 x10 2 Pa Tsfc 278.7456K R 287 J / K kg and then use the above equation: v800 RTsfc 287J / K kg 278.7456K 1.000m 3 2 p800 800x10 Pa At 800 hPa, the parcels have the same pressure (mechanical equilibrium), but the isothermal parcel has a larger volume because it has a higher temperature. Heat has been added to the isothermal parcel, while the adiabatic parcel is allowed to cool as it rises. (b) For the parcel that rises isothermally, derive an expression for the time rate of change of the specific volume as a function of the vertical p-velocity , where dp . dt T is constant, so dv d RT RT dt dt p p2 2. (a) Show that d c p d ln for a reversible processes. Take the ln of the definition of potential temperature, and then the differential: R p cp R R T 0 ln ln T ln p 0 ln p d ln T d ln d ln p cp cp p Derive and expression for dlnT from the first law: dT dq vdp dq RTdp dq c p dT vdp dq d ln T d ln p T c p T c p T c p T c p pT c p T Comparing the 2 equations above, d ln dp dq dp dq d ln c p d ln d p cpT p cpT (b) How did the specific entropy of Parcel A change in moving from the surface to 500 hPa in Question 1 above? How did the specific entropy of Parcel B change? For parcel A, doesn’t change. For parcel B, d c p d ln sfc Tsfc 278.7K p 800 T800 0 p800 R cp 1000 278.8K 800 287 1004 297.2K d 1004J / K kg ln 800 ln 1000 64.53J / K kg (c) Were the changes in specific entropy found in (b) above consistent with Claussius’ inequality? Explain briefly. Yes, since the change of entropy was zero (Parcel A) or positive (Parcel B). 3. Is the dependence of pressure on geometric height (depth) in the troposphere similar to that in the ocean? Explain briefly. No. Pressure decreases exponentially with height in the atmosphere, but increases linearly with depth in the oceans. 4. Why is cp > cv? Possibly Useful Formulas and Constants acceleration due to gravity (g) = 9.81 m/s2 Stefan-Boltzman constant () = 5.67 x 10-8 W/m2-K4 solar “constant” (So) = 1370 W/m2 q q cp = =1004 J/kg-K cv = = 717 J/kg-K T v T p cp - cv = R gas constants Rd = dry air: 287 J/K-kg Rv = water vapor: 461 J/K-kg density (0˚ C, 1000 mb) air=1.275 kg m-3 water=1.000 x 103kg m-3 sand = 2.65 x 103kg m-3 q c p dT vdp q cv dT pdv q dh vdp R p cp T 0 p q T 0 d ln q cp T q d T rev cp cv h = u+pv R 0.286 cp Poisson’s Equations: Tv 1 constant Tp constant The Maxwell Relations: T p v s s v s p v T T v T pv p s s p s v T p p T pv constant