Determination of an Equilibrium Constant
advertisement

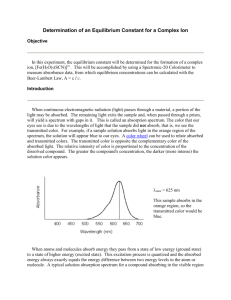
DETERMINATION OF AN EQUILIBRIUM CONSTANT Introduction In this experiment, we will be dealing with the reaction between the ferric or iron (III) ion, (Fe3+) and the thiocyanate ion (SCN-). The reaction is shown below. Fe(SCN)2+ Fe3+ + SCN- colorless red-brown color You will determine the equilibrium constant at various initial concentrations of iron (III). You will use the UV-vis spectrophotometer to determine the concentration of Fe(SCN)2+ in your solutions. The UV-vis spectrophotometer measures the amount of light absorbed at each wavelength in the ultraviolet and visible region of the light spectrum. The absorbance of light in the visible region is proportional to the concentration of the colored component. You have probably seen this technique used to estimate the strength of tea or coffee. The darker the color, the more concentrated the flavoring. The UV-vis is much more quantitative than your eyes at measuring concentration (absorbance). Goals: During this activity, students will 1. use the UV-vis to measure the absorbance in the visible region of solutions containing different concentrations of iron thiocyanate. 2. use the absorbance measurements to calculate the concentration of iron thiocyanate. 3. determine the concentration of each component. 4. calculate an equilibrium constant for the reaction between iron (III) ions and thiocyanate ions. Prelab Questions 1. What is the source of iron (III) ions in solution for this experiment? ______________ What is the source of thiocyanate ions in solution for this experiment? ________________ 2. (a) Write the complete ionic equation for the reaction. (b) What are the spectator ions? (c) Write the net ionic equation. (d) What type of reaction is this? Exp. 20 Page 1 3. (a) What is the color of the Fe(NO3)3 solution? Do you expect it to absorb light in the visible region? Support your answer. (b) What is the color of the KSCN solution? Do you expect it to absorb light in the visible region? Support your answer. 4. Write the equilibrium constant expression for the system shown in question 2c. Safety: Wear safety goggles in the lab. If you get any solutions on you, wash with soap and water. Materials: UV-vis and computer kimwipes plastic cuvet 1 mL volumetric pipet 10 mL volumetric pipet 2 mL pipet pump 25 mL Erlenmeyer (5/group) 50 mL beaker (for waste) 10 mL pipet pump 0.2 M Fe(NO3)3/ 0.5 M HNO3 solution 0.001 M Fe(NO3)3/ 0.5 M HNO3 solution 0.001 M KSCN solution 0.5 M HNO3 solution Procedure: Preparation of solutions 1. Record the exact molarity of the two iron solutions and the thiocyanate solution below. Exact concentration of 0.2 M Fe3+ __________________ Exact concentration of 0.001 M Fe3+ _________________ Exact concentration of SCN- __________________ Exp. 20 Page 2 2. Prepare solutions 1 through 5 using the amounts in the chart below. Use the volumetric pipets to measure your volumes as accurately as possible. Be sure you mark your container so you know which solution is which. Solution HINTS FOR USING VOLUMETRIC PIPETS Do not pull the solution into the pipet pump. If you do, any residue in the pump will contaminate future solutions. Pay attention to the measurement scale. You should never completely empty the pipet into the solution because the last portion of the pipet is not scaled. Hold the pipet and the pipet pump carefully. Otherwise a leak may occur, or worse—you may drop it! When measuring multiple solutions, make all measurements for one solution before proceeding to the next solution. This minimizes the risk of contamination. Always rinse the pipet with the new solution by pulling some into the pipet and releasing into a waste container. This way, you are coating the sides of the pipet with the new solution. Then measure your desired volume. mL 0.2 M Fe(NO3)3 mL 0.001 M Fe(NO3)3 mL 0.001 M KSCN mL 0.5 M HNO3 1 9 0 1 0 2 3 0 1 6 3 1 0 1 8 4 .5 0 1 8.5 5 0 6 1 3 The UV-vis: Collecting reference and dark spectra 3. Mark the top edge of one face of your cuvet using a permanent marker. 4. Fill the cuvet 1/2 full with your blank solution (water). 5. Wipe off the outside of the blank cuvet with the special paper provided. This will remove fingerprints, dripped isopropanol, and other substances that may interfere with the analysis by absorbing light. 6. Insert the cuvet into the sample port of the instrument and note the position of your marking. In the future, always insert your cuvet the same way. Make sure the cuvet is completely inserted. In some instruments, there will be some resistance before the cuvet is all the way in. Exp. 20 Page 3 7. Press Reference and then Store. 8. Remove the cuvet. 9. Insert the black cuvet into the sample port. This blocks light from entering the fiber optic cable. 10. Press Dark and then Store. 11. Remove the black paper. You are now ready to collect your data!!! The UV-vis: Collecting Data 12. Using a pipette, rinse the cuvet with your solution. Then fill the cuvet 1/2 full with the solution and wipe the cuvet with the special paper. 13. Place the cuvet in the sample port, making sure the marking is oriented the same as it was for your background. 14. Press Scan 15. Save your file by selecting Save Spectral Values under the File menu. Type in a name for the spectrum and click OK. Record the name of your file in your data table. 16. Determine the absorbance at 455 nm by clicking the black + button in the window in the upper right. Then click the red crosshairs in the graph window and trace along the spectrum to approximately 455 nm. The wavelength and absorbance are printed in the upper right corner. To fine-tune the wavelength, press the left or right diamond of the bigger diamond in the upper right window. Record the value for the absorbance at 455 nm in your data table. 17. Using a pipette, remove the solution from the cuvet and dispose of it in the waste beaker. 18. Repeat steps 12-17 for each solution to be analyzed. Printing your data 19. To view all your spectra on the screen at once, select file and Open Spectrum. Select one of your saved files from the list and press Open. Keep track of which file is which color in the table below. Repeat until you have opened all your files. 20. Print your spectra. Clean-up 21. Rinse the cuvets several times with distilled water. 22. Dispose of your solutions in the designated waste containers. Exp. 20 Page 4 23. Wash your hands with soap and water. Data Table Solution Absorbance @ 455 nm Color of Spectrum Name of Saved File 1 2 3 4 5 Postlab Calculations: 1. In these calculations, we are trying to determine the equilibrium constant in each solution 2-5. Rewrite the equilibrium constant expression for the reaction that occurred. 2. What are the three components of the reaction? 3. In solution 1, the initial concentration of Fe3+ is about 1800 times the initial SCNconcentration. We can then assume that virtually all of the thiocyanate is converted to Fe(SCN)2+. Therefore, the equilibrium concentration of Fe(SCN)2+ is equal to the initial concentration of SCN-. For example, the initial concentration of the KSCN can be calculated by M1V1 = M2V2 (1 mL stock)(mol arity stock) (10 mL total volume) (a) Calculate the initial concentration of KSCN in solution 1. (b) What is the equilibrium concentration of Fe(SCN)2+? _______________ 4. The absorbance at 455 nm is due completely to Fe(SCN)2+. What is the absorbance in solution 1 at 455 nm? _______________ What is the concentration of Fe(SCN)2+ in solution 1? ______________ Exp. 20 Page 5 5. Since not all complexes absorb the same amount of light/concentration, we need to determine a constant to tell how much light Fe(SCN)2+ absorbs at 455 nm. We can calculate this constant (c) using solution 1 and the equation below. [Fe(SCN)2+] = (c)(Absorbance @ 455 nm) Calculate the constant for your system. c = ___________ 6. For solutions 2-5, you need to use your constant to measure the equilibrium concentrations of Fe(SCN)2+. We are going to start with solution 2. First, find the initial concentrations of each of the components. Reminder: initial means that no reaction has occurred. So, the initial concentration of Fe(SCN)2+ is zero. Fill in the initial concentrations for solution 2 in the table below. Fe3+ SCN- Initial FeSCN2+ 0 Change Final/Equilibrium 7. Although you don't know your equilibrium concentration of Fe(SCN)2+, you do know the absorbance at 455 nm from Fe(SCN)2+ and the constant to relate absorbance to concentration. Calculate the equilibrium concentration of Fe(SCN)2+. 8. Remember, the equation we are using is: Fe(SCN)2+ Fe3+ + SCN- colorless red-brown color For every one mole of Fe(SCN)2+ produced, one mole of Fe3+ and one mole of SCN- reacts. Use this information to fill in the remainder of the table. Exp. 20 Page 6 9. Now that you have the equilibrium concentrations of all the substances in the reaction, calculate the equilibrium constant. 10. On separate paper, repeat the calculations for solutions 3-5. 11. How did your lab support what you know about equilibrium systems (e.g. LeChatelier's Principle)? 12. What are the possible sources of error in this experiment? Exp. 20 Page 7