Experiment 20 - Determination of the equilibrium constant for the
advertisement

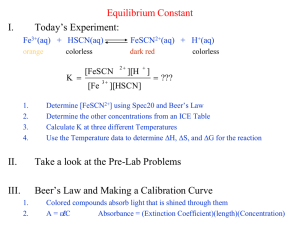
Experiment 19 - Determination of the equilibrium constant for the reaction Fe3+ (aq) + SCN (aq) = FeSCN2+ (aq) Object: To determine the equilibrium constant by a colorimetric method Theory: Colorimetric methods of analysis are usually applied to the determination of small concentrations of either inorganic or organic materials in a solution. The constituent sought must be coloured or must be capable of reacting with a reagent to produce a substance having a suitable colour. Beers Law, which relates the degree of absorption of monochromatic light to the concentration of an absorbing substance in a solution; is combined with Lambert's and Bouguer's relationships of absorption to length of the light path, to form the following exponential expression. I I 0 e kCT where I = intensity of light transmitted by the absorbing layer I 0 = intensity of light incident on the absorbing layer C = concentration of substance in absorbing layer T = thickness of absorbing layer k = constant of proportionality This combined expression is frequently called "Beers Law" and may be re-written in the form log( The log ratio of I0 ) kCT I I0 is known as absorbance and has no unit. This term is also called optical density and is the quantity that the I instrument measures and displays on the meter. As the thickness of the absorbing layer is maintained constant by examining the solution in standard test tubes, the value of absorbance A is proportional to the concentration of the solution. I0 ) I A kCT (1) A log( If Beers Law holds for a range of concentrations of a solution, as A is directly proportional to the concentration. AC Beer’s Law Hence A1 C1 A2 C2 (2) where C1 = concentration of unknown C2 = concentration of standard A1 = absorbance of unknown A2 = absorbance of standard It is usually to construct a calibration curve of absorbance against concentration. If Beers Law holds for the solutions investigated the curve will be a straight line passing through the origin. The reaction under consideration here is the formation of complex ions: Fe3+ (aq) + SCN (aq) ====== FeSCN2+ (aq) For colour determination, one standard solution of FeSCN2+ can be prepared by adding a small concentration of SCN (aq) to a large excess of Fe3+ (aq) so that essentially all the SCN (aq) is converted to FeSCN2+ (aq). Under this conditions, the final concentration of FeSCN2+ (aq) in the standard solution is equal to the concentration of SCN (aq) solutions used. The equilibrium concentration of FeSCN2+ (aq) in each mixture is determined by comparison with the above standard solution. The equilibrium concentrations of Fe3+ (aq) and SCN (aq) are obtained by subtracting [FeSCN2+] formed from the initial [Fe3+] and [SCN]. At equilibrium: K= [ FeSCN 2 ] [ Fe3 ][SCN ] Chemicals: 0.2 M iron (III) nitrate, 0.002 M potassium thiocyanate Apparatus: colorimeter, burette, test-tubes Procedure: 1. Line up five clean test tubes, all of the same size, and label them A, B, C, D, E, F and G. 2. Run the liquids, according to the following given table into the conical flask. Shake well and then allow the flask to stand at room temperature for a few minutes, to allow the mixture to reach equilibrium. The total volume for each experiment is 10 cm 3. Experiment Fe3+ / cm3 water/ cm3 SCN / cm3 A 5 0 5 B 5 5 0 C 0.5 5 4.5 D 0.4 5 4.6 E 0.3 5 4.7 F 0.2 5 4.8 G 0.1 5 4.9 3. Insert a test tube A in the colorimeter, and close the light cover over the tube. 4. Turn on the instrument and adjust the rotary sensitivity control to bring the meter to full scale deflection (i.e. 100% Transmission). It is advisable to check this setting frequently as drift may occur. If the control requires serious alteration, previous measurements must be repeated. 5. Turn off the instrument, remove the tube A and replace with the tube B. Re-close the cover. 6. Turn on the instrument again and note the new reading in absorbance on the meter scale. 7. Repeat step 5 - 6 for the tube C, D, E, F and G. 8. Record the room temperature. Result: Room temperature = _________ C Absorbance of standard (Tube B) = _______________ [FeSCN2+] of standard (Tube B) = _______________ Absorbance [FeSCN2+]eq [Fe3+]eq [SCN]eq Tube C Tube D Tube E Tube F Tube G Calculation: 1. Consider the tube B as standard. Calculate [FeSCN2+] of the standard. 2. Calculate the equilibrium concentration of FeSCN2+ (aq) for tube C to F by using the equation (2). 3. Calculate the equilibrium concentration of Fe3+ (aq) ion, by subtracting the equilibrium concentration of FeSCN2+ (aq) ion from the initial concentration of the Fe3+ (aq) ion. 4. Calculate the equilibrium concentration of SCN (aq) ion in the same manner as for the Fe3+ (aq) ion. Subtract the equilibrium concentration of the FeSCN2+ (aq) ion from the initial concentration of the SCN (aq) ion. 5. Find the equilibrium constant for each test-tube. Question: 1. What is colorimetry? What is its limitation in application? 2. Suggest a method to prepare the reaction mixture of tube C, D, E, F and G. 3. State the approximation in this experiment. 4. Plot a graph of [FeSCN2+] against [Fe3+][ SCN] and find the equilibrium constant from the graph. 5. Discuss the deviation of Beers Law.