Analysis of an Unknown Chloride by Titration
advertisement

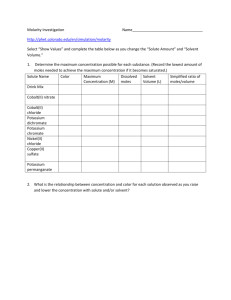
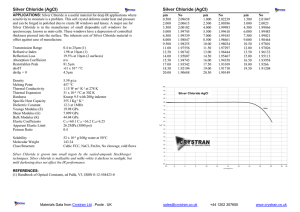
Analysis of an Unknown Chloride by Titration Marjorie Carosiello TL Hanna High School Anderson, SC September 26, 2011 Titration is a volumetric procedure where by the identity of an unknown can be determined by adding precise quantities of a standard solution of know concentration using a buret. The moles of the unknown may be calculated when the endpoint is reached as the moles of the known solution equal the moles of the unknown. This information allows the calculation of the molarity, or mass of the unknown dependant on the circumstances. In this lab an unknown group I chloride was titrated with 0.200 M silver nitrate, potassium chromate was used to indicate the endpoint. The solution turned cream and then buff to indicate that all of the silver ions had precipitated with the chloride ions and a second (red) precipitate, silver chromate began to fall out of solution. The reactions are listed below: Ag+ (aq) + Cl- (aq) --> AgCl(s) 2Ag+(aq) + CrO4-2(aq) ----> Ag2CrO4 (s) Reaction 1 Reaction 2 The unknown chloride was found to have 39.65, 38.57, 38.51 g/mol in the three samples respectively. The conclusion of the experimenter was the identity of the unknown was potassium. The actual mass is reported as 39.01 g/mol. The per cent error was determined to be 0.513%. This study illustrates that titration can be effective in quantitative analysis. Grading Title Author and address Topic/question Method Results Conclusion +5 +5 +10 +25 +25 +20 points Ea missing rxn Ea incorrect sig dig Ea incorrect unit Neatness -5 -1 -1 -10 The scale above is based on a 90 pt lab and a 10 pt prelab

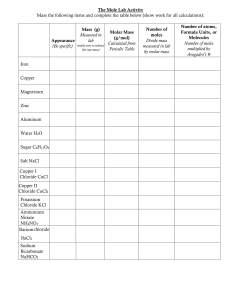









![Solubility Equilibria Assignment Question [2005/RI/III/3] The](http://s2.studylib.net/store/data/010161949_1-86b5ffaf7f49fedf26a2dad1aba191ff-300x300.png)