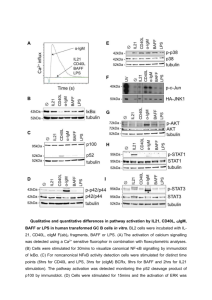
Effect of NF-kB Transcription Inhibition in E6 and E7 Expressing
advertisement

Effect of NF-B Transcription Inhibition in E6 and E7 Expressing Tumor Lines Theresa Hopkins Problem: The search for new cancer treatments is a continual process. While there are many effective treatments already available, there is a constant search for ways to improve upon these existing treatments. Cancer cells do not respond normally to the body's control mechanisms, and do not respond to cell-cycle controls such as apoptosis (planned cell death). These cells do not exhibit density dependent inhibition as normal cells do, and will divide excessively and indefinitely. Some varieties of cancer are caused by a virus, which will interfere with cellular mechanisms, causing the unresponsiveness to cell-cycle controls. The human papilloma virus, which causes both cervical cancer and benign warts, induces NF-B transcription and prevents apoptosis, making it resistant to programmed cell death, and thus allowing it to grow and spread. By determining the causes and effects of this type of inhibition, new cancer treatments can be discovered, and existing treatments can be made more effective. Literary Background: NF-B has become a widely researched area, with a wide variety of experiments performed. The involvement of the NF-B in causing a variety of human diseases, including cancer, diabetes, and AIDS has been investigated (I). The mechanism for NF-B activation has been discovered, with an emphasis on the importance of the inhibitory protein, IB- (2). NF-B pathway appropriation by viruses has also been investigated for a number of viruses, including HPV, HIV, and EBV (Epstein-Barr virus). These viruses manipulate the NFB pathway for many reasons, including but not limited to the inhibition of apoptosis, enhancement of viral replication, and evasion of immune responses (3). Activation of NF-B in cancer cells by viruses, chemotherapy, or radiation treatments has been found to reduce the effectiveness of anti-cancer treatments, and the regulation ofthe NF-B cycle has become a logical therapy target for certain cancers (4). In papillomaviruses, the E6 and E7 viral proteins have been identified as the factor that influences the NF-B transcription cycle, and the interaction between these proteins and cell growth regulators offers a target site fur therapeutic developments (5). Goal: To test whether inhibition of NF-B activity will alter apoptosis or growth of HPV-16 E6/E7 expressing cells. Background: The transcription factor NF-B has been connected with multiple aspects of oncogenesis, including the cell cycle, differentiation, cell migration, and the control of apoptosis. In cells where NF-B is inactive, activity is inhibited by the IB- protein, which binds the NF-B protein complex and prevents it from translocating to the nucleus. When the inhibitory protein is degraded by phosphorylation, the NF-B complex is released to the nucleus, where it stimulates gene transcription. Target genes are selectively regulated by the distinct transcriptional potential of different combinations of NF-B proteins. In Human Papilloma Virus 16 (HPV-16) cells expressing the viral E6 and E7 genes E6/E7 can trigger the phosphorylation and degradation of the IB- protein by interfering with cellular retinoblastoma and p53 proteins, thereby activating NF-B. E6 and E7 are retained and expressed in most cervical carcinomas, and their continual expression is necessary to maintain a malignant phenotype. The importance of NF-B expression in the survival of E6/E7 expressing cells is unclear. By transfecting E6/E7 expressing cells with a dominant IB- mutant that is resistant to phosphorylation and degradation, NF-B transcription can be halted, and the effects of decreased NF-B on the survival of E6/E7 expressing cells can be determined. Hypothesis: NF-B activation by E6/E7 is important to minimize the susceptibility of infected cells to apoptosis. Project outline: In order to determine the importance of NF-B, we will cotransfect cervical epithelial cells with the dominant negative IB- mutant, the -galactosidase reporter gene, and either HPV-16 E6, E7, or E6 and E7. A -gal assay will then be performed to determine the amount of NF-B activity. DAPI stains will also be performed in order to determine the extent of cellular damage and the amount of apoptosis. The controls used in this experiment will be cells that are transfected with vector-only DNA in place of E6/E7 and/or the IB- mutant. Also, in order to determine if NF-B activity is actually decreased, the NF-B reporter gene will be substituted for -galactosidase in selected experiments. References: 1. Baldwin, Jr., Albert S. “The Transcription Factor NF-B and Human Disease.” Journal of Clinical Investigation 107 (2001): 3-6. 2. Baldwin, Jr., Albert S. "The NF-B and IB Proteins: New Discoveries and Insights. II Annual Review of Immunology 14 ( 1996): 649-683. 3. Genin, Pierre, John Hiscott, and Hakju Kwon. "Hostile Takeovers: Viral Appropriation of the NF-B Pathway.” Journal of Clinical Investigation 107 (2001): 143-151. 4. Baldwin, Jr., Albert S. "Control of Oncogenesis and Cancer Therapy Resistance By the Transcription Factor NF-B." The Journal of Clinical Investigation 107 (2001): 241-246. 5. Farthing, Alan I., and Karen H. Vousden. "Functions of Human Papillomavirus E6 and E7 Proteins.” Trends in Microbiology 2 (1994): 170-174.