subject Chemistry
advertisement

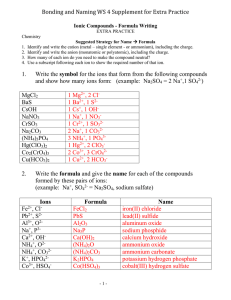
Tests on the discipline of Chemistry 1. What are the two ions are not p-electrons in the ground state? a) Li + and Na + b) H + and Li + c) Li + and Fd) Br- and F2. Ionic radius decreases in the series a) Na + -Mg2 + -Al3 + b) Mg2 + -Ca2 + -Sr2 + c) O2 - S2 - Se2d) F - O2 - N33. Are the following statements concerning the properties of aluminum and its compounds? A. Aluminum hydroxide decomposes when heated to form an oxide. B. The highest degree of oxidation of aluminum is equal to three. a) A true only b) Is only true B c) Both statements are true d) Both statements are incorrect 4. Covalent bonds have a connection: a) Na2S b) CaO c) KI d) CH3ONa 5. The sulfur atom has a negative oxidation state in the compound: a) SCl2 b) FeS2 c) CH3ONa d) SF6 6. The crystal lattice of fullerene C60: a) Nuclear b) Ionic c) Molecular d) Metal 7. In the list of substances A) C5H10 (OH) 2 B) C2H2O2 In) C3H7OH D) C2H6O2 D) C2H4O E) C3H6 (OH) 2 By limiting the dihydric alcohols include a) AE b) BG c) AVD d) AGE 8. The common property of nitrogen and phosphorus - their ability to interact with a) Magnesium b) sulfuric acid c) iron d) carbon 9. What oxide reacts with the water, but does not interact with the solid calcium hydroxide? a) an oxide of phosphorus (V) b) sodium oxide c) the silicon oxide (IV) d) aluminum oxide 10. Ca (OH) 2 is convert to CaCO3, on reaction with a) C b) CO c) CO2 d) HCOOH 11. The chemical reaction takes place between a) calcium nitrate and sodium carbonate b) sodium sulfate, and barium nitrate c) sodium hydrogen sulfate solution and ammonia d) with sodium sulfate and ammonia 12. The circuit of transformations: 1. +X +Y Cu2O ——→ Cu ——→ CuCl2 Determine substances X and Y. a) X-Ag, Y-FeCl2 b) X-H2, Y-HCl c) X-CO2, Y-AgCl d) X-CO, Y-HgCl2 13. In which connection all the carbon atoms are in the same hybrid state. a) HC≡C-C≡CH b) CH3-CH = CH2 c) CH3-CH = O d) CH3-CH = CH-CH3 14. The isomerization reaction is possible for a) Methane b) Ethane c) Propane d) Pentane 15. Are the following statements about the polyhydric alcohol? A. Ethylene glycol and glycerol were dissolved Cu (OH) 2. B. Lower polios infinitely miscible with water. a) A is true only b) is true only B c) Both statements Verna d) Both statements are incorrect 16. Ammoniac solution of silver oxide does not react a) Formaldehyde b) Ethanol c) Glucose d) Formic acid 17. The passage of vapor propanol-1 over hot copper oxide is form: a) Propanol b) Acetone c) The methyl ethyl ketone d) Propene 18. In the scheme of reactions: C6H6 → X → C6H5COOH Substance X a) S6H5CH3 b) C6H5Cl c) C6H5OH d) C6H12 19. The interaction of benzene with chlorine in the light - this is a reaction a) Substitutions b) Connections c) Cleavage d) Exchange 20. Dimerization reaction of nitric oxide (IV) 2NO2 (g) = N2O4 (g) - Elementary. Pressure in the system increased by 6 times. How many times will increase the rate of the forward reaction? a) 6 b) 12 c) 36 d) 216 21. What is the reaction with water is reversible under normal conditions? a) CH3COOCH3 + H2O = CH3COOH + CH3OH b) 2Na + 2H2O = 2NaOH + H2 c) CaO + H2O = Ca (OH) 2 d) SO3 + H2O = H2SO4 22. In which of the molar solution concentration of H + ions greatest? a) HCl b) HClO4 c) H2SO4 d) CH3COOH 23. In alkaline solution does not react a) Magnesium sulfate b) Silver nitrate c) Sodium hydrogen carbonate d) Calcium carbonate 24. Unknown salt stains flame of a spirit lamp in purple, under the action of hydrochloric acid by a yellow-green suffocating gas. When heated in the presence of a catalyst salt is decompose to form gas that breaks smoldering splinter. Determine salt. a) NaNO3 b) KNO3 c) KClO3 d) K2SO3 25. What material prepared using benzene and ethylene as starting material? a) Toluene b) Rubber c) Polyvinyl acetate d) Polystyrene