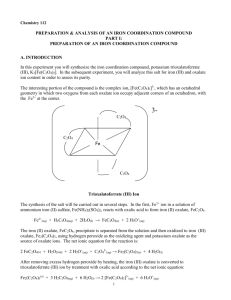
Synthesis & Analysis of a Complex Iron Salt
advertisement

Determination of the % Water in a Complex Salt Crystal In the previous experiment a green crystalline product having the formula KxFe(C2O4)y . zH2O was prepared. In the next few experiments beginning with this one, the percentage composition of the compound will be determined, and from that information, the empirical formula will be derived. Finally, the percent yield will be determined. The green iron oxalato complex is one of a number of solid chemicals that are classified as "hydrates". Familiar examples of hydrates are Plaster of Paris (CaSO4.1/2 H2O), gypsum, (CaSO4.2 H2O), and alum [KAl(SO4) 2.12 H2O]. The water of hydration of many hydrates can be removed as a gas by heating the hydrate to a temperature above 100oC for a period of time. The following reaction involving barium chloride dihydrate (BaCl2.2 H2O) occurs when the solid is heated above 100oC. BaCl2.2 H2O(s) BaCl2 (s) + 2 H2O (g) The percentage of water of hydration in KxFe(C2O4)y.zH2O will be determined in this experiment by heating a weighed sample of green hydrate in an open container in a drying oven until all of the water of hydration has been driven off. KxFe(C2O4)y . zH2O KxFe(C2O4)y + z H2O (g) The loss in weight will be set equal to the mass of the water of hydration. Objective: To determine the percentage of water of hydration in the compound KxFe(C2O4)y . zH2O. Materials: Electronic balances, two 150 mL beakers, desiccator, beaker tongs, crucible tongs, watch glass, crucibles & covers, hot plate or drying oven, green complex salt crystals Safety, Environmental, and Economic Concerns: Waste chemicals from this experiment may be safely discarded in the trash. Tips for Experimental Procedures: 1. You must perform two trials using no more than 2.0 g total of your green complex salt crystals. 2. Always have crucible lids set slightly ajar while they are being heated, as a buildup of pressure inside the crucible can make it go flying if not vented. The crucible should be completely covered, however, for drying and massing times. 3. After they have been heated, the crucibles & covers should not be touched by fingers until the experiment is completed due to the moisture and oils your hands may impart. Handle the crucible & cover with crucible tongs. 4. The most frequent cause of erratic balance readings is failure to have objects at room temperature when they are being weighed. Convection currents in a closed balance compartment can have a surprising effect. 5. While cooling crystals a dessicator is a good piece of equipment to employ since it prevents humidity from reabsorbing into the crystals. To protect the dessicator from spills, all crucibles should be placed into beakers before being placed in the dessicator. After the final massing, empty the dried crystals into the trash. DO NOT MIX THE ANHYDROUS CRYSTALS WITH THE ORIGINAL CRYSTALS! Determination of the % Oxalate in a Complex Salt Crystal The percentage oxalate in KxFe(C2O4)y . zH2O will be determined by performing a redox titration. The mass and percentage of oxalate in the sample can be determined by measuring the volume, V, of ~0.01 M KMnO4 required to completely oxidize the oxalate ion (C2O4-2). KMnO4 oxidizes C2O4-2 in acid solution to give CO2 gas as a product. 16 H+ + 5 C2O4-2 + 2 MnO4- ------------> 10 CO2 + 2 Mn+2 + 8 H2O The stoichiometry of this reaction requires an oxalate/permanganate ion ratio of 5 : 2, or stated differently, 5 moles of oxalate ion are chemically equivalent to 2 moles of the permanganate ion. In this reaction it follows that the number of moles of oxalate ion in the sample is: moles C2O4-2= (V x M) x 5/2 V : volume of KMnO4 required to react with C2O4-2; M : molarity of KMnO4 solution The mass and percentage of oxalate in a weighed sample of the green salt is calculated from: mass of oxalate in sample = (5/2)(V)(M)(FW) FW : Formula Weight of Oxalate % C2O4-2 = [(mass of C2O4-2)/(mass of green salt sample)] x 100 With the completion of this experiment, the percent composition of the green salt, KxFe(C2O4)y . zH2O, has been completely determined experimentally. The simplest (empirical) formula can be calculated from the % composition. Once the formula is known it is possible to calculate the percent yield obtained in the original synthesis reaction. Objective: To determine the percentage of oxalate ion in KxFe(C2O4)y . zH2O. Apparatus: Electronic balance, spatula, two 250 mL Erlenmeyer flasks, buret, buret clamp, ring stand, 25 mL graduated cylinder, two 50 mL beakers, green complex salt crystals, ~0.01 M KMnO4 solution, concentrated H3PO4 solution, 6 M H2SO4 Safety, Environmental, and Economic Concerns: All waste solutions from this experiment should be poured in the waste container under the fume hood. Tips for Experimental Procedures: 1. The oxidation state of iron in KxFe(C2O4)y . zH2O is +3, normally the highest value for iron. Thus the KMnO4 does not oxidize the iron in this experiment. However, the presence of the ferric ion imparts a yellow color to the solution. The H3PO4 forms a colorless phosphate complex with the iron, making it easier to detect the color change which occurs at the equivalence point. 2. The reaction of the permanganate ion with the oxalate ion is rather slow at room temperature. Heating the solution increases the rate of the reaction. 3. Use no more than 0.125g green salt crystal, 6 mL sulfuric and 1 mL phosphoric acid in each sample. 4. Calculate the empirical formula and the percent yield of your crystal based on the percentages obtained throughout these analytical experiments. Save the remaining crystals in the amber bottle for the next series of experiments!
![[Zr(C 2 O 4 ) 4 ] 4](http://s3.studylib.net/store/data/006964769_1-29aedaf41342f4132b60bdeb351827c4-300x300.png)