211-12Acid-Base
advertisement

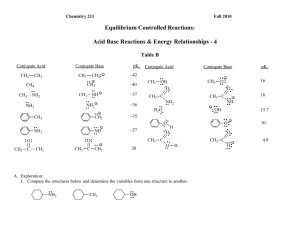
Chemistry 211 Fall 2012 Equilibrium Controlled Reactions: Acid Base Reactions & Energy Relationships - 4 Table B Conjugate Acid CH2: CH3 CH4 NH - ~35 NH : CH3 CH3 C CH3 CH3 20 C O A. Exploration: 1. Compare the structures below and determine the variables among the structures. CH3 : : : NH2 OH 10 :O : CH3 C H 16 15.7 O: - : : C CH2 : OH O: H : : : O: :O : :O : ~27 NH : : : : : NH2 - H2O 16 :O : NH2 O - O: CH3 C : : : : : : :O : CH3 C ~36 CH2 CH3 ~37 CH3 OH : : : : : NH2 - NH3 ~40 CH3 pKa : : CH3 NH2 : : : CH3 Conjugate Base ~42 : CH3 Conjugate Acid : : CH3 pKa : : CH3 Conjugate Base 4.8 Acid-Base Rxns & Energy Relationships-4 2 2. Use the data in Table B to list the compounds in 1. in the order of decreasing acidity and provide a warrant citing specific data in Table B that support your claim. (As usual, more data supporting a warrant makes the claim stronger.) Most Acidic Middle Least acidic Warrant: 3. Which of the following ions has the lowest energy? Which has the highest energy? Provide a warrant citing specific data in Table B that support your claims. (As usual, more data supporting a warrant makes the claim stronger.) O Lowest Energy Warrant: NH C CH2 : O: C : : - : : C O O Highest Energy Acid-Base Rxns & Energy Relationships-4 3 B. Using The Data in Table B, our General Principles and Relative Effect assumption, suggest theoretical backing to explain the effects observed above in terms of electron nuclear attraction and/or electron-electron repulsion. Acid-Base Rxns & Energy Relationships-4 4 C. In Class Applications: 1. Using the theoretical backing developed in Part B, select the most acidic proton(s) in the following compound. Then provide a warrant describing the process and logic that led from the theory to your claim. H H H H C C H H C H N C O C C H H H H H H 2. Use the theoretical backing in this activity to predict whether the equilibrium constant for the following reaction should be > or < 1. Provide a warrant describing the process and logic that led from the theory to your claim. O + NH2 : NH : : H + : : O : : : Acid-Base Rxns & Energy Relationships-4 5 Reflector’s Report Discussion: Identify the most important concepts you learned from this activity: What questions remain? Strategy Analyst’s Report Discussion: A. Exploration: What role did question 1 play in the exploration of Table B in question 2? What Principle or Assumption that wasn’t necessary for question 2 was required to answer question 3? B. Theoretical Backing: What fundamental aspect of the variable identified in question A. 1. was the key to providing the backing needed in B.? How was it used?