class xi autumn break home work 2015-16
advertisement

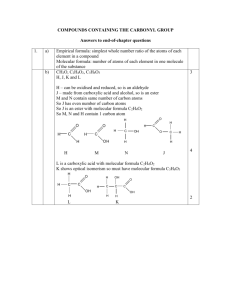
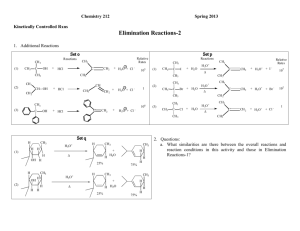
K V Khammam Vacation Home Work (Autumn Break) 2015-16. Class: XI ________________________________________________________________ Sub: English 1. Read at least two short reports in any English newspaper. Cut and paste them on your note-book. On the basis of your reading of these reports, make notes on them in points only, using headings and sub-headings. Use recognizable abbreviations wherever necessary. 2. Write a speech in about 150 words about the Increasing Crime rate against Women. 3. During the Autumn break, a team of school students from Kendriya Vidyalaya, Khammam visited a village named Raghunatha Puram . The team was much worried on noticing the most pitiable insanitary conditions prevailing there. The team collected the villagers and its leader Mr. Ram Yadav and gave a short speech on the necessity and benefits of remaining clean. Write the speech in about 200 words. 4. Write a letter in not more than 150-200 words to the Editor of a daily commenting on the increasing display of violence in latest movies. 5. Praveen Chandra of 38-G, Gokulpuri, Palam, New Delhi wants to let out a portion of his house only to foreigners/ Central Govt. employees. Draft a suitable advertisement to be published in the To-Let column of a national daily giving necessary details. 6. Two Unseen passages : Write/ paste the passages in note book and solve the passage questions. 7. Reading Task: Read the prescribed novel “ The Canterville Ghost” and write the book review . Focus on characters and theme. CHEMISTRY HOLIDAYS HOMEWORK 1.For the equilibrium, 2NOCl(g) ⇌ 2NO(g) + Cl2 (g) the value of the equilibrium constant, Kc is 3.75 × 10–6 at 1069 K. Calculate the Kp for the reaction at this temperature? 2. The following concentrations were obtained for the formation of NH3 from N2 and H2 at equilibrium at 500K. [N2 ] = 1.5 × 10–2M. [H2 ] = 3.0 ×10–2 M and [NH3 ] = 1.2 ×10–2M. Calculate equilibrium constant. 3. What will be the conjugate bases for the following Brönsted acids: HF, H2 SO4 and HCO3 – ? 4. Write the conjugate acids for the following Brönsted bases: NH2 – , NH3 and HCOO – . 5. Classify the following species into Lewis acids and Lewis bases and show how these act as such: (a) HO – (b)F – (c) H+ (d) BCl3 6. The concentration of hydrogen ion in a sample of soft drink is 1x 10–3M. What is its pH ? 7. Describe the effect of a) addition of H2 b) addition of CH3OH c) removal of CO d) removal of CH3OH on the equilibrium of the reaction: 2H2 (g) + CO (g) ⇌CH3OH (g) 8. Explain the physical significance of van der Waals parameters. 9. 34.05 mL of phosphorus vapour weighs 0.0625 g at 546 °C and 0.1 bar pressure. What is the molar mass of phosphorus? 10. What will be the pressure of the gaseous mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in a 1L vessel at 27°C? 11. A neon-dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If pressure of the mixture of gases in the cylinder is 25 bar. What is the partial pressure of dioxygen and neon in the mixture ? 12. At 25°C and 760 mm of Hg pressure a gas occupies 600 mL volume. What will be its pressure at a height where temperature is 10°C and volume of the gas is 640 ml. 13. Draw the Lewis structures for the following molecules and ions : H2S, SiCl 4 , BeF2 , CO3 −2 , HCOOH 14. Discuss the shape of the following molecules using the VSEPR model: BeCl2 , BCl3 , SiCl4 , AsF5 , H2S, PH3 15. Which hybrid orbitals are used by carbon atoms in the following molecules? CH3 –CH3; (b) CH3 –CH=CH2; (c) CH3 -CH2 -OH; (d) CH3 -CHO (e) CH3 COOH 16. Describe the hybridization in case of PCl5 . Why are the axial bonds longer as compared to equatorial bonds? 17. Assign oxidation number to the underlined elements in each of the following species: (a) NaH2PO4 (b) NaHSO4 (c) H4P2O7 (d) K2MnO4 (e) CaO2 18. Balance the following equations in basic medium by ion-electron method and oxidation number methods and identify the oxidizing agent and the reducing agent. (a) P4(s) + OH– (aq) (b) N2H4(l) + ClO3- (aq) PH3(g) + HPO2 – (aq) NO(g) + Cl– (g) (c) Cl2O7 (g) + H2O2(aq) ClO2 – (aq) + O2(g) + H+ 19. Depict the galvanic cell in which the reaction Zn(s) + 2Ag+ (aq) Zn2+(aq) +2Ag(s) takes place, Further show: (i) which of the electrode is negatively charged, (ii) the carriers of the current in the cell, and (iii) individual reaction at each electrode. 20. Given the standard electrode potentials, K+ /K = –2.93V, Ag+ /Ag = 0.80V, Hg2+/Hg = 0.79V Mg2+/Mg = –2.37V. Cr3+ /Cr = –0.74V arrange these metals in their increasing order of reducing power विषय-हिन्दी 1) परियोजना कायय – स्वर साम्राज्ञी लता मंगेशकर जी के व्यक्ततत्व पर प्रकाश डालते हुए जीवनवत ृ ललखिए तथा उनके फिल्म जगत में दिये योगिान पर सचित्र प्रकाश डाललए | 2) समनद ु े शन कायय- संतलु लत भोजन के महत्व पर प्रकाश डालते हुए एक िीिर ललखिए | 3) फकसी िै ननक समािार पत्र के संपािक को सड़क िर् घ नाओं को रोकने ु ट के ललए सझ ु ाव िे ते हुए एक पत्र ललखिए | 4) ‘गलता लोहा’ पाठ के आधार पर ग्रामीण बच्िों की लशक्षा के ववषय पर ननबंध ललखिए |