pola27008-sup-0001-suppinfo01
advertisement
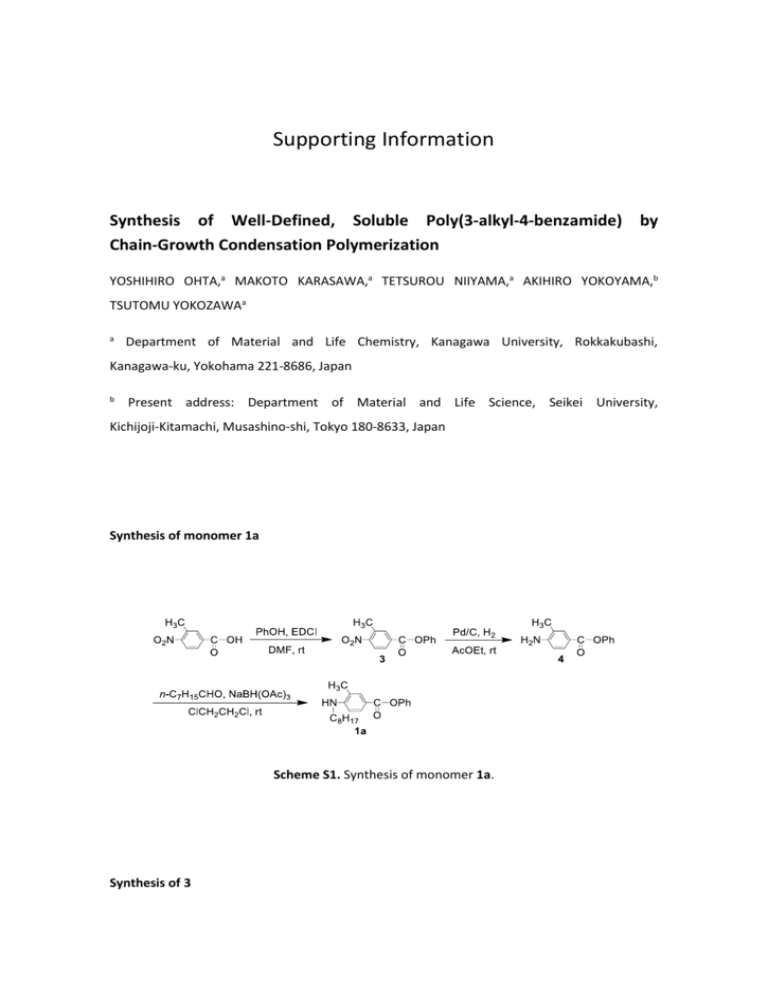
Supporting Information Synthesis of Well-Defined, Soluble Poly(3-alkyl-4-benzamide) by Chain-Growth Condensation Polymerization YOSHIHIRO OHTA,a MAKOTO KARASAWA,a TETSUROU NIIYAMA,a AKIHIRO YOKOYAMA,b TSUTOMU YOKOZAWAa a Department of Material and Life Chemistry, Kanagawa University, Rokkakubashi, Kanagawa-ku, Yokohama 221-8686, Japan b Present address: Department of Material and Life Science, Seikei University, Kichijoji-Kitamachi, Musashino-shi, Tokyo 180-8633, Japan Synthesis of monomer 1a Scheme S1. Synthesis of monomer 1a. Synthesis of 3 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) (5.84 g, 30.5 mmol) was added to a solution of 3-methyl-4-nitrobenzoic acid (5.03 g, 27.8 mmol), 4-(dimethylamino)pyridine (DMAP) (4.08 g, 33.3 mmol) and phenol (3.14 g, 33.4 mmol) in dry DMF (120 mL) at 0 oC. The mixture was stirred at ambient temperature for 19.5 h, and then the reaction was quenched with water. The whole was extracted with CH2Cl2. The combined organic layers were washed with 1 M HCl, water, and brine, dried over anhydrous MgSO4, and concentrated in vacuo. The residue was recrystallized from ethyl acetate-hexane to give 3 as a yellow plate crystal (4.18 g, 59%). mp: 96.5-97.5 oC. H NMR (600 MHz, CDCl3, , ppm): 8.19 (s, 1 H), 8.15 (dd, J = 8.5 and 1.6 Hz, 1 H), 8.03 (d, J = 1 8.5 Hz, 1 H), 7.45 (t, J = 8.1 Hz, 2 H), 7.31 (t, J = 7.4 Hz, 1 H), 7.22 (d, J = 7.5 Hz, 2 H), 2.67 (s, 3 H). C NMR (150 MHz, CDCl3, , ppm): 163.5, 152.2, 150.5, 134.5, 133.6, 133.2, 129.6, 128.6, 13 126.3, 124.7, 121.4, 20.1. IR (KBr): 3120, 1735, 1586, 1522, 1491, 900, 850, 744, 693 cm-1. Synthesis of 4 Ethyl acetate (80 mL), 3 (4.07 g, 15.8 mmol), and 5% Pd/C (0.265 g) were placed in a flask. The atmosphere in the flask was replaced with hydrogen. The mixture was stirred at room temperature for 2.5 h, and then filtrated through Celite®. The filtrate was concentrated under reduced pressure, and the residue was purified by flash chromatograph on silica gel (hexane/ethyl acetate = 2/1) to give 4 as a white solid (2.45 g, 68%). mp: 104.0-106.0 oC. H NMR (600 MHz, CDCl3, , ppm): 7.91 (s, 1 H), 7.89 (s, 1 H), 7.40 (t, J = 7.9 Hz, 2 H), 7.23 (t, J = 1 9.1 Hz, 2 H), 7.18 (d, J = 7.6 Hz, 2 H), 6.69 (d, J = 8.3 Hz, 1 H), 4.09 (s, 2 H), 2.21 (s, 3 H). 13C NMR (150 MHz, CDCl3, , ppm): 165.4, 151.3, 149.8, 132.9, 130.1, 129.3, 125.4, 121.9, 121.1, 118.6, 113.7, 17.1. IR (KBr): 3489, 3388, 3058, 1704, 1487, 906, 746, 692 cm-1. Synthesis of 1a Sodium triacetoxyborohydride (3.00 g, 14.2 mmol) was added to a solution of 4 (3.00 g, 13.2 mmol) and octanal (2.0 mL, 13 mmol) in 1,2-dichloroethane (40 mL) at room temperature. The mixture was stirred for 3 h, and then the reaction was quenched with sat. NaHCO3. The whole was extracted with CH2Cl2. The organic layer was washed with water, dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatograph on silica gel (hexane/ethyl acetate = 10/1) to give 1a as a white solid (2.20 g, 49%). mp: 58.0-59.0 oC. H NMR (600 MHz, CDCl3, , ppm): 7.99 (dd, J = 6.5 and 1.0 Hz, 1 H), 7.88 (d, J = 1.2 Hz, 1 H), 1 7.40 (t, J = 8.5 Hz, 2 H), 7.23 (t, J = 7.4 Hz, 1 H), 7.18 (d, J = 8.5 Hz, 2 H), 6.61 (d, J = 8.6 Hz, 1 H), 4.02 (br s, 1 H), 3.25 (q, J = 7.2 Hz, 2 H), 2.17 (s, 3 H), 1.69 (quint, J = 7.2 Hz, 2 H), 1.46-1.24 (m, 10 H), 0.89 (t, J = 7.0 Hz, 3 H). 13C NMR (150 MHz, CDCl3, , ppm): 165.6, 151.4, 150.7, 132.1, 130.6, 129.3, 125.3, 121.9, 120.6, 116.4, 108.3, 43.5, 31.8, 29.34, 29.32, 29.2, 27.1, 22.6, 17.2, 14.1. IR (KBr): 3413, 2961, 2928, 2854, 1710, 1576, 1495, 902, 739, 683 cm-1. Synthesis of monomer 1b Scheme S2. Synthesis of monomer 1b. Synthesis of 7 A solution of iodine chloride (10.0 g, 61.7 mmol) in acetic acid (200 mL) was added dropwise over 0.5 h to a solution of 61 (13.0 g, 61.0 mmol) in acetic acid (850 mL) at ambient temperature. The mixture was stirred for 3.5 h and quenched with sat. NaHCO3. The whole was extracted with CH2Cl2. The organic layer was washed with sat. NaHCO3 and brine, dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatograph on silica gel (hexane/CH2Cl2 = 1/1) to give 7 as a light yellow solid (17.5 g, 85%). mp: 124.0-125.4 oC. H NMR (600 MHz, CDCl3, , ppm): 8.49 (d, J = 1.7 Hz, 1 H), 7.97 (dd, J = 8.2 and 2.1 Hz, 1 H), 1 7.41 (t, J = 7.9 Hz, 2 H), 7.25 (t, J = 7.6 Hz, 1 H), 7.18 (d, J = 8.8 Hz, 2 H), 6.76 (d, J = 8.6 Hz, 1 H), 4.62 (br s, 2 H). 13C NMR (150 MHz, CDCl3, , ppm): 163.7, 151.1, 150.8, 141.5, 131.7, 129.3, 125.6, 121.6, 120.1, 113.0, 81.9. IR (KBr): 3462, 3358, 1702, 1623, 1198, 1158, 875, 835, 744, 691 cm-1. Synthesis of 8 A three-necked flask, equipped with a three-way stopcock, was dried at 250 oC under reduced pressure. The flask was cooled to room temperature under argon atmosphere. Bis(triphenylphosphine)palladium(II) dichloride (1.03 g, 1.47 mmol), 7 (10.01 g, 29.5 mmol), triphenylphosphine (0.777 g, 2.96 mmol), copper iodide (0.565 g, 2.97 mmol), Et3N (10 mL, 72 mmol), and dry DMF (280 mL) were placed in the flask. The flask was degassed, and then 1-octyne (4.08 g, 37.0 mmol) was added. The mixture was stirred at ambient temperature for 5.5 h, and then the reaction was quenched with sat. NH4Cl. The whole was extracted with ether. The organic layer was washed with brine and water, dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatograph on silica gel (hexane/ethyl acetate = 5/1) three times, and then recrystallized from ethyl acetate-hexane to give 8 as a light yellow solid (4.01 g, 42%). mp: 105.3-107.0 oC. H NMR (500 MHz, CDCl3, , ppm): 8.12 (d, J = 2.0 Hz, 1 H), 7.91 (dd, J = 8.5 and 2.2 Hz, 1 H), 1 7.40 (t, J = 7.4 Hz, 2 H), 7.24 (t, J = 7.4 Hz, 1 H), 7.18 (d, J = 8.7 Hz, 2 H), 6.70 (d, J = 8.3 Hz, 1 H), 4.68 (br s, 2 H), 2.48 (t, J = 7.2 Hz, 2 H), 1.63 (quint, J = 7.4 Hz, 2 H), 1.47 (quint, J = 7.4 Hz, 2 H), 1.36-1.30 (m, 4 H), 0.91 (t, J = 7.0 Hz, 3 H). 13C NMR (125 MHz, CDCl3, , ppm): 164.8, 152.0, 151.1, 134.8, 131.2, 129.3, 125.5, 121.8, 118.1, 113.0, 108.2, 96.6, 75.8, 31.3, 28.7, 28.6, 22.5, 19.5, 14.0. IR (KBr): 3461, 3344, 3210, 2932, 1697, 1507, 919, 745, 721, 690 cm-1. Synthesis of 9 Methanol (1.1 L), 8 (3.78 g, 11.8 mmol), and 5% Pd/C (1.38 g) were placed in the flask. The atmosphere in the flask was replaced with hydrogen. The mixture was stirred at room temperature for 4 h, and then filtrated through Celite®. The filtrate was concentrated under reduced pressure. The residue was purified by flash chromatograph on silica gel (hexane/ethyl acetate = 5/1), and then recrystallized from hexane and methanol to give 9 as a white solid (2.54 g, 66%). mp: 63.1-64.2 oC. H NMR (600 MHz, CDCl3, , ppm): 7.90-7.88 (m, 2 H), 7.41 (t, J = 7.7 Hz, 2 H), 7.24 (t, J = 7.4 Hz, 1 1 H), 7.19 (d, J = 7.9 Hz, 2 H), 6.69 (d, J = 8.6 Hz, 1 H), 4.11 (br s, 2 H), 2.51 (t, J = 7.9 Hz, 2 H), 1.66 (quint, J = 7.7 Hz, 2 H), 1.40 (quint, J = 7.3 Hz, 2 H), 1.37-1.25 (m, 8 H), 0.88 (t, J = 6.9 Hz, 3 H). 13C NMR (150 MHz, CDCl3, , ppm): 165.5, 151.3, 149.3, 131.8, 129.8, 129.3, 125.6, 121.9, 118.6, 114.2, 31.8, 31.0, 29.6, 29.4, 29.2, 28.3, 22.6, 14.0. IR (KBr): 3389, 2928, 2847, 1709, 1238, 1194, 929, 740 cm-1. Synthesis of 10 A solution of octanoyl chloride (1.34 g, 8.24 mmol) in dry CH2Cl2 (8.0 mL) was added dropwise to a solution of Et3N (0.851 g, 8.41 mmol) and 9 (1.80 g, 5.53 mmol) in dry CH2Cl2 (10 mL) at 0 oC under dry nitrogen. The mixture was stirred at ambient temperature for 3 h, and a solution of octanoyl chloride (0.462 g, 2.84 mmol) in dry CH2Cl2 (2.0 mL) was added again dropwise to the mixture at 0 oC under dry nitrogen. The mixture was stirred at room temperature for 0.5 h, and then the reaction was quenched with water. The whole was extracted with CH2Cl2. The organic layer was washed with 1 M HCl, sat. NaHCO3, and water, dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatograph on silica gel (hexane/CH2Cl2 = 1/2) to give 10 as a white solid (2.24 g, 90%). mp: 113.5-115.2 oC. H NMR (600 MHz, CDCl3, , ppm): 8.25 (br s, 1 H), 8.06 (dd, J = 8.6 and 2.1 Hz, 1 H), 8.00 (d, J = 1 2.1 Hz, 1 H), 7.42 (t, J = 8.6 Hz, 2 H), 7.26 (t, J = 7.6 Hz, 1 H), 7.21 (d, J = 8.6 Hz, 2 H), 7.20 (d, J = 8.6 Hz, 1 H), 2.63 (t, J = 7.9 Hz, 2 H), 2.43 (t, J = 7.4 Hz, 2 H), 1.76 (quint, J = 7.5 Hz, 2 H), 1.65 (quint, J = 7.6 Hz, 2 H), 1.43-1.38 (m, 4 H), 1.36-1.25 (m, 14 H), 0.89 (t, J = 6.5 Hz, 3 H), 0.88 (t, J = 6.7 Hz, 3 H). 13C NMR (150 MHz, CDCl3, , ppm): 171.5, 164.8, 150.9, 140.2 131.3, 129.4, 129.0, 125.7, 125.0, 121.9, 121.7, 37.9, 31.8, 31.6, 31.3, 29.5, 29.48, 29.40, 29.2, 29.0, 25.6, 22.6, 22.5, 14.02, 14.00. IR (KBr): 3267, 2955, 2919, 1737, 1525, 1282, 1191, 1133 840, 743, 689 cm-1. Synthesis of 1b A solution of 10 (2.04 g, 4.52 mmol) in dry THF (50 mL) was added dropwise to a two-necked flask containing 1.0 M borane-THF complex (9.1 mL, 9.1 mmol) at 0 oC with stirring under dry nitrogen. The mixture was stirred under reflux for 3.5 h, and allowed to cool to ambient temperature. 6 M HCl (9.0 mL) was added to the flask, and then the mixture was stirred under reflux for 1 h. The reaction mixture was cooled to room temperature and poured into water. The mixture was neutralized with sat. NaHCO3, and then the whole was extracted with ethyl acetate. The organic layer was washed with water, dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel (hexane/CH2Cl2 = 2/1) to give 1b as a white solid (1.20 g, 61%). mp: 52.1-53.3 oC. H NMR (600 MHz, CDCl3, , ppm): 7.98 (dd, J = 8.6 and 2.1 Hz, 1 H), 7.86 (d, J = 2.1 Hz, 1 H), 1 7.38 (t, J = 7.6 Hz, 2 H), 7.21 (t, J = 7.6 Hz, 1 H), 7.18 (d, J = 7.6 Hz, 2 H), 6.60 (d, J = 8.6 Hz, 1 H), 4.12 (br s, 1 H), 3.20 (br s, 2 H), 2.30 (t, J = 7.9 Hz, 2 H), 1.68-1.61 (m, 4 H), 1.43-1.37 (m, 4 H), 1.35-1.26 (m, 16 H), 0.89 (t, J = 6.9 Hz, 3 H), 0.88 (t, J = 6.9 Hz, 3 H). 13C NMR (150 MHz, CDCl3, , ppm): 165.6, 151.4, 150.2, 131.0, 130.4, 129.2, 125.2, 125.1, 121.9, 116.4, 108.6, 43.4, 31.8, 31.7, 30.8, 29.6, 29.4, 29.3, 29.2, 28.1, 27.1, 22.6, 14.0. IR (KBr): 3407, 2925, 1702, 1285, 1159, 742, 690 cm-1. Synthesis of phenyl 4-(trifluoromethyl)benzoate 2b Scheme S3. Synthesis of phenyl 4-(trifluoromethyl)beozoate 2b. Synthesis of phenyl 4-(trifluoromethyl)benzoate 2b A solution of 4-(trifluoromethyl)benzoyl chloride (5.00 g, 24.0 mmol) in dry CH2Cl2 (5.0 mL) was added dropwise to a solution of Et3N (4.50 mL, 32.3 mmol) and phenol (2.38 g, 25.3 mmol) in dry CH2Cl2 (20 mL) at 0 oC under dry nitrogen. The mixture was stirred at ambient temperature for 3 h, and then the reaction was quenched with water. The whole was extracted with CH2Cl2. The organic layer was washed with 1 M HCl, sat. NaHCO3, and water, dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was purified by recrystallization from hexane and methanol to give 2b as a white solid (5.11 g, 80%). mp: 92.3-94.1 oC (lit.2 90-92 oC). H NMR (600 MHz, CDCl3, , ppm): 8.32 (d, J = 7.9 Hz, 2 H), 7.78 (d, J = 8.2 Hz, 2 H), 7.45 (t, J = 1 7.6 Hz, 2 H), 7.30 (t, J = 7.6 Hz, 1 H), 7.22 (d, J = 7.6 Hz, 2 H). 13C NMR (150 MHz, CDCl3, , ppm): 164.0, 150.6, 135.0 (q, J = 32.5 Hz), 132.8, 130.6, 129.6, 126.2, 125.6 (q, J = 4.3 Hz), 123.5 (q, J = 273 Hz), 121.5. 19F NMR (565 MHz, CDCl3, , ppm) 98.70. IR (KBr): 1736, 1593, 1491, 1325, 864, 744, 696 cm-1. Synthesis of monomer 1c Scheme S4. Synthesis of monomer 1c. Synthesis of 11 A solution of 4-octyloxybenzoic acid (4.62 g, 18.4 mmol) in thionyl chloride (25 mL) was stirred at ambient temperature for 40 h and then concentrated in vacuo. The residual acid chloride was dissolved in CH2Cl2. A solution of Et3N (1.87 g, 18.5 mmol) and 9 (4.02 g, 12.3 mmol) in dry CH2Cl2 (20 mL) was placed in a two-necked flask equipped with a three-way stopcock, to which the solution of acid chloride was added dropwise at 0 oC under dry nitrogen. The mixture was stirred at room temperature for 4 h, then the reaction was quenched with water. The whole was extracted with CH2Cl2. The organic layer was washed with 1 M HCl, sat. NaHCO3 and water, and then dried over anhydrous MgSO4. The solvent was removed in vacuo, and the residue was purified by flash chromatography on silica gel (hexane/CH2Cl2 = 2/3) two times to give 11 as a white solid (2.86 g, 42%). mp: 117.7-120.8 oC. H NMR (600 MHz, CDCl3, , ppm): 8.41 (d, J = 8.6 Hz, 1 H), 8.11 (dd, J = 8.6 and 2.1 Hz, 1 H), 1 8.04 (d, J = 2.1 Hz, 1 H), 7.91 (s, 1 H), 7.84 (d, J = 8.6 Hz, 2 H), 7.43 (t, J = 8.1 Hz, 2 H), 7.27 (t, J = 7.2 Hz, 2 H), 7.22 (d, J = 8.6 Hz, 2 H), 7.00 (d, J = 8.9 Hz, 2 H), 4.03 (t, J = 6.7 Hz, 2 H), 2.72 (t, J = 7.7 Hz, 2 H), 1.83 (quint, J = 7.0 Hz, 2 H), 1.74 (quint, J = 7.7 Hz, 2 H), 1.48 (quint, J = 7.6 Hz, 2 H), 1.43 (quint, J = 7.4 Hz, 2 H), 1.39-1.20 (m, 16 H), 0.90 (t, J = 6.9 Hz, 3 H), 0.87 (t, J = 7.0 Hz, 3 H). C NMR (150 MHz, CDCl3, , ppm): 165.0, 164.9, 162.4, 151.0, 140.6, 131.6, 131.5, 129.4, 13 129.3, 128.8, 126.5, 125.7, 124.9, 121.8, 121.4, 114.6, 68.3, 31.8, 31.5, 29.51, 29.45, 29.4, 29.3, 29.19, 29.16, 29.1, 26.0, 22.62, 22.59, 14.07, 14.06. IR (KBr): 3448, 2921, 2852, 1733, 1507, 1281, 1266, 1252, 1174, 1065, 841, 741, 689 cm-1. Synthesis of 1c A solution of 11 (1.96 g, 3.52 mmol) in dry THF (50 mL) was added dropwise to a two-necked flask containing 1.15 M borane-THF complex (6.15 mL, 7.07 mmol) at 0 oC with stirring under dry nitrogen. The mixture was stirred under reflux for 4 h, and allowed to cool to ambient temperature. 6 M HCl (6 mL) was added to the flask, and then the mixture was stirred under reflux for 1 h. The reaction mixture was cooled to room temperature and poured into water. The mixture was neutralized with sat. NaHCO3, and then the whole was extracted with ethyl acetate. The organic layer was washed with water and sat. NaHCO3, dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel (hexane/ethyl acetate = 15/1) two times to give 1c as a white solid (1.75 g, 91%). mp: 48.6-50.1 oC. H NMR (600 MHz, CDCl3, , ppm): 7.96 (dd, J = 8.6 and 2.1 Hz, 1 H), 7.88 (d, J = 2.1 Hz, 1 H), 1 7.40 (t, J = 8.6 Hz, 2 H), 7.27 (d, J = 8.6 Hz, 1 H), 7.23 (t, J = 7.6 Hz, 1 H), 7.19 (d, J = 8.6 Hz, 2 H), 6.90 (d, J = 8.6 Hz, 2 H), 6.65 (d, J = 8.6 Hz, 1 H), 4.43 (br s, 1 H), 4.38 (s, 2 H), 3.95 (t, J = 6.5 Hz, 2 H), 2.49 (t, J = 7.7 Hz, 2 H), 1.78 (quint, J = 7.1 Hz, 2 H), 1.66 (quint, J = 7.6 Hz, 2 H), 1.45 (quint, J = 7.5 Hz, 2 H), 1.41-1.23 (m, 18 H), 0.89 (t, J = 7.1 Hz, 3 H), 0.88 (t, J = 7.2 Hz, 3 H). 13C NMR (150 MHz, CDCl3, , ppm): 165.6, 158.6, 151.3, 149.9, 131.0, 130.4, 130.0, 129.3, 128.6, 125.4, 125.3, 121.9, 117.1, 114.7, 109.2, 68.0, 47.3, 31.82, 31.78, 30.9, 29.6, 29.4, 29.3, 29.24, 29.20, 28.1, 26.0, 22.6, 14.1. IR (KBr): 3432, 2926, 2855, 1722, 1513, 1283, 1246, 1196, 1136, 1070, 822, 742, 689 cm-1. REFERENCES 1 T. Yokozawa, M. Ogawa, A. Sekino, R. Sugi, A. Yokoyama, J. Am. Chem. Soc. 2002, 124, 15158-15159. 2 D. A. Watson, X. Fan, S. L. Buchwald, The Journal of Organic Chemistry 2008, 73, 7096-7101.









