Purpose
advertisement

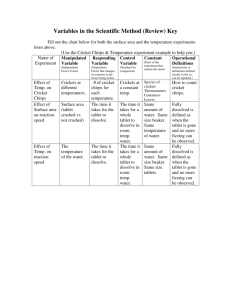
Lab: Examining Reaction Rate & Temperature Purpose: Several factors affect the rate of a chemical reaction. This lab allows you to examine the effect of temperature on a common chemical reaction. Hypothesis: How do you think temperature will affect the rate at which a chemical reaction happens? Materials: Alka-Seltzer tablet small beaker (250 mL) hot plate, balance Styrofoam cup 100 mL graduated cylinder tap water thermometer stop watch graph paper Procedure: 1. Measure ~ 200 mL of TAP WATER in a 250 mL beaker and start heating it. 2. Take a single Alka-Seltzer tablet and break it into four roughly equal pieces (or multiple pieces that can be combined to make four roughly equal samples). 3. Measure the mass of one piece of the tablet and record it in the data table. 4. Measure 50 mL of tap water from the faucet (using the 100 mL graduated cylinder). Pour this tap water into a Styrofoam cup and mark the inside of the cup with a line to mark 50 mL. 5. Measure the temperature of this water (to 1 decimal place). Record temperature in the data table. 6. With a stop watch ready, add the piece of tablet to the water. Record the amount of time elapsed between when the tablet hits the water and when you see that all the pieces of tablet have dissolved in the water. 7. Repeat steps 3 – 6 twice more. EXCEPT, use water temperatures of about 50oC and 65oC (the exact temperatures will be recorded in your data table when you do that trial). ** YOU WILL DO THE 4th PIECE IN ANALYSIS QUESTION #5 ON THE BACK. SAVE THE 4th PIECE!!! ** Data Table: Temperature (oC) Mass (g) Time (sec) Reaction Rate (g/sec) Analysis: 1. Calculate the reaction rate by finding the mass/time for each trial. Record these values in the data table. ** SHOW YOUR CALCULATIONS ** 2. Graph the reaction rate (mass/time) vs temperature for the trials. The reaction rate should be on the y-axis and the temperature should be on the x-axis. Draw a trend line (a straight line through your data, averaging your trials) Don’t forget a descriptive title & labels on your axis (with units) 3. What is the relationship between reaction rate and temperature for this reaction? 4. Using your graphed data and trend line, PREDICT the reaction rate for the reaction carried out at 40oC. 5. LAB: Heat 50 mL of water to ~ 40oC. Using the last piece of your Alka-Seltzer tablet, repeat steps 3 – 6 of the procedure. Record the data in the data table and calculate the reaction rate (g/sec) 6. How did your prediction for the reaction rate at 40oC compare to the actual reaction rate? What are three possible sources of error?