Chemistry Year 11 Ideas Paper June 2012 Practise Questions
advertisement

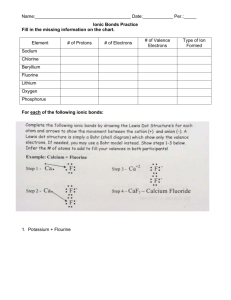
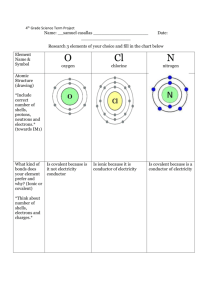
Chemistry Year 11 Ideas Paper June 2012 Practise Questions 1) Which group of the periodic table are the halogens? 2) What are the similarities in electron structure of the halogens? 3) How many protons, neutrons & electrons do the Chlorine and Bromine have? 4) Which period is Chlorine in? 5) Which period Bromine in? 6) How do the halogens form ions? 7) Draw the electron arrangements of Chlorine and Bromine? 8) Draw the ions of Chlorine and Bromine? 9) Group 1 metals form +1 ions. Write the formulas of: a. Potassium bromide b. Sodium chloride c. Lithium iodide d. Potassium Chloride e. Lithium bromide 10) What type of bonding occurs between the halogens and group 1 metals? 11) Describe the type of structure formed and state the properties of these types of structures. 12) What colour and state are chlorine, bromine & iodine at room temperature? 13) State the formula of chlorine and bromine molecules. 14) Draw a dot-cross diagram to show the bonding in a molecule of chlorine and state the type of bond formed. 15) What is the pattern of reactivity as you descend (go down) the group? 16) Explain this pattern of reactivity in terms of electrons. 17) Why do the halogens show similar chemical characteristics? 18) Describe the trend as you go down the group in: a. Melting point b. Boiling point c. Density 19) Using the table on page 4, decide what state chlorine and bromine would be in at: a. -110°C b. -50°C c. -10°C d. 25°C e. 70°C 20) What are the hazards of chlorine and bromine? 21) Explain why chlorine is hazardous to transport over large distances. 22) Chlorine is made by electrolysis of brine (Salty water) like in the diagram above. The salt in brine is sodium chloride. The ions present when salt is dissolved in water are H+, OH-, Na+ & Cl-. Chlorine ions go to the positive electrode and form chlorine gas. The hydrogen ions go to the negative electrode and form hydrogen gas. The remaining sodium and hydroxide ions react to form sodium hydroxide. Write half equations to show the reactions that occur at each electrode. 23) Explain why ionic compounds conduct electricity when molten or dissolved in water but not when solid. 24) Research the uses of sodium hydroxide, chlorine and hydrogen produced from the electrolysis of brine. 25) Bromine is made from sea water using displacement reactions. Chlorine gas is bubbled through the solution containing the halogen compound. If chlorine is more reactive than the halogen in the compound the chlorine will bond with the non-halogen element and leave the halogen on its own. For example: Sodium bromine + chlorine sodium chloride + bromine a. Predict the outcomes of the following reactions: i. Sodium iodide + chlorine ii. Potassium fluoride + chlorine iii. Lithium bromide + chlorine iv. Sodium fluoride + chlorine v. Potassium iodide + chlorine b. For the reactions that occur above, write balanced symbol equations, including state symbols (note that the halogen compounds are solutions, the halogens that are produced will dissolve in water and the chlorine is bubbled through the solution).

![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)