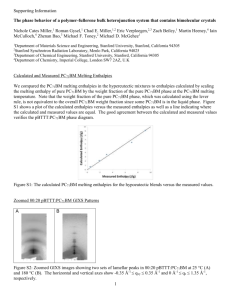
Name, last name Expt 1 melting point analysis Date of experiment
advertisement

Name, last name
Partner name, last name
Expt 1 melting point analysis
Date of experiment
Purpose: To use melting point analysis for the determination of a pure unknown
Procedure: As in Chem 230 lab manual, Camosun College, Revised July 2008 by Dr. Nasr
Khalifa, pages 1-4.
Report format is tabular and answer all questions at the end of the lab (no need for purpose and
procedure here).
Data and Results
Table 1 Melting point analysis of known organic compounds
Compound
Mpt oC lab
Mpt oC lit1
Structure
Urea
Cinnamic acid
Table 2. Melting point of a mixture of urea and cinnamic acid
Mixture (~50/50)
Actual mpt.
Expected mpt.
Table 3 Determination of the unknown (LETTER) by melting point analysis
Known
+ Unknown (LETTER)
Mpt oC lab
Mandelic acid
Benzoic acid
{discussion is not required}
Discussion The melting point of pure compounds are narrow and sharp. This will be lower with
a wider range with the presence of impurities. By first determining the melting point of a pure
unknown and then mixing it with a known compound with fairly similar melting points, the
identity of the unknown can be determined. The melting point with a narrow and sharp range
will be that of the pure compound. Refer to tables created in report (ie, Table 1 summarizes the
melting point of different organic compounds). Sources of error which may cause differences
between experimental results and literature include the following: Fast heating of the thiele
tube which causes overheating of the samples and missed melting points, impurities
inadvertently added to pure compounds or unknowns (supposedly pure). These impurities may
have been added by previous students, and pure samples may not have been powered which
causes slower melting of the compound in crystalline form.
Conclusion Unknown Letter has.,.{one sentence- no need to add further material}.
References
1. R.Raap, N.Khalifa. Organic Chemistry Experiments for Chemistry 230 and 231.
Camosun College, Department of Chemistry and Geoscience, Victoria, BC, Revised
July 2005. pages 43-45.
2. Wikipedia, the free encyclopedia http://en.wikipedia.org/wiki/. Accessed May 15, 2008.
3. McMurry, J. Organic Chemistry, 7th ed.: Brooks Cole, 2008.