Test study guide here
advertisement

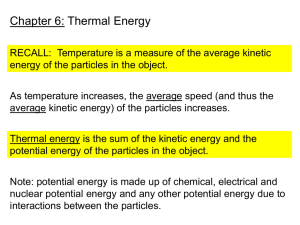
Investigation 5 Practice ’13-Z Investigation 5 Practice ’13-Z Write your answers to the following on a separate piece of paper. Bring with you to the retake session you scheduled. 1. Explain how energy transfers between objects of the same temperature 2. Explain how energy transfers between objects of different temperatures 3. Energy “flow” is the transfer of energy from one place to another. Which direction does energy flow? 4. What equation is used to calculate the final temperature when equal masses of water are mixed? 5. What equation is used to calculate how much heat energy (calories) is transferred to or from a mass of water? Write your answers to the following on a separate piece of paper. Bring with you to the retake session you scheduled. 3. Explain how energy transfers between objects of the same temperature 4. Explain how energy transfers between objects of different temperatures 3. Energy “flow” is the transfer of energy from one place to another. Which direction does energy flow? 4. What equation is used to calculate the final temperature when equal masses of water are mixed? 5. What equation is used to calculate how much heat energy (calories) is transferred to or from a mass of water? A plastic bottle filled with warm air is submerged in cold water. 1. What direction does energy flow to cool the air inside the bottle 2. List the materials the energy would have to flow through. 3. How does energy transfer actually happen to cool the air inside the bottle? 4. How long does the energy transfer happen? A plastic bottle filled with warm air is submerged in cold water. 5. What direction does energy flow to cool the air inside the bottle 6. List the materials the energy would have to flow through. 7. How does energy transfer actually happen to cool the air inside the bottle? 8. How long does the energy transfer happen? Write the 3 formulas for finding final temp., change in temp. and calories transferred. Write the 3 formulas for finding final temp., change in temp. and calories transferred. Stephen mixed 25 mL of water at 15°C and 25 mL of water at 65°C. Calculate the answers to the following questions. For each one, list the formula, plug in the numbers (with units) and record the final answer. Stephen mixed 25 mL of water at 15°C and 25 mL of water at 65°C. Calculate the answers to the following questions. For each one, list the formula, plug in the numbers (with units) and record the final answer. What is the final temperature of the water? What is the change in temperature of the water? How much heat energy was transferred from the hot water? How much heat energy was transferred to the cold water? What is the final temperature of the water? What is the change in temperature of the water? How much heat energy was transferred from the hot water? How much heat energy was transferred to the cold water? When a student mixes 50 mL of water at 15 ºC with 50 mL of water at 80ºC, what happens to the particles? When a student mixes 50 mL of water at 15 ºC with 50 mL of water at 80ºC, what happens to the particles? The warm water particles _________________ with the cold water particles The warm water particles start to move _____________ and in the __________________ direction The cold water particles start to move _____________ and in the _______________ direction The warm water particles _________________ with the cold water particles The warm water particles start to move _____________ and in the __________________ direction The cold water particles start to move _____________ and in the _______________ direction How much heat energy transferred from the hot water to the cold water (3 steps) How much heat energy transferred from the hot water to the cold water (3 steps) A student has two identical glasses of milk except that the temperature of the milk in one glass is 15 ºC and the temperature of the milk in the other glass is 80ºC. The glass of milk at which temperature has more thermal energy? Explain why… A student has two identical glasses of milk except that the temperature of the milk in one glass is 15 ºC and the temperature of the milk in the other glass is 80ºC. The glass of milk at which temperature has more thermal energy? Explain why… A student has two glasses of milk at the same temperature. The only difference is that one glass is ½ full and the other is ¾ full. The glass of milk at which glass has more thermal energy? Explain why… Multiple Choice – choose your answer and then SUPPORT with reasoning or evidence why your answer is correct…. The temperature of a plastic cup is 20ºC. It is filled with water that is 10ºC. Which of the following describes how thermal/heat energy is transferred? Support your answer A. Heat energy is transferred from the water to the cup until they are both at 15ºF. B. Heat energy is transferred from the cup to the water until they are both at 15ºF. C. Heat energy is transferred from the cup to the water until the cup is at 10ºC and the water is at 20ºC. D. No heat energy is transferred between the cup and the water, so the cup will stay at 20ºC and the water will stay at 100ºC. An empty paper cup is the same temperature as the air in the room. A student fills the cup with cold water. Which of the following describes how thermal/heat energy is transferred? Support your answer A. Thermal/heat energy is transferred from the cold water to the cup until they are at the same temperature. B. Thermal/heat energy is transferred from the cup to the cold water until they are at the same temperature. C. Thermal/heat energy is transferred from the cup to the cold water until the cup has no more thermal energy. D. Thermal/heat energy is not transferred between the cup and the cold water. A student is holding a cold piece of metal in her hand. While she is holding the piece of metal, her hand gets colder. Does the piece of metal get warmer? Why or why not? Support your answer A. Yes, the piece of metal will get warmer because some thermal energy is transferred from the metal to the student’s hand. B. Yes, the piece of metal will get warmer because some thermal energy is transferred from the student’s hand to the metal. C. No, the piece of metal will stay at the same temperature because an equal amount of thermal energy is exchanged between the student’s hand and the metal. D. No, the piece of metal will stay at the same temperature because thermal energy is not transferred between the student’s hand and the metal. A student has two glasses of milk at the same temperature. The only difference is that one glass is ½ full and the other is ¾ full. The glass of milk at which glass has more thermal energy? Explain why… Multiple Choice – choose your answer and then SUPPORT with reasoning or evidence why your answer is correct…. The temperature of a plastic cup is 20ºC. It is filled with water that is 10ºC. Which of the following describes how thermal/heat energy is transferred? Support your answer A. Heat energy is transferred from the water to the cup until they are both at 15ºF. B. Heat energy is transferred from the cup to the water until they are both at 15ºF. C. Heat energy is transferred from the cup to the water until the cup is at 10ºC and the water is at 20ºC. D. No heat energy is transferred between the cup and the water, so the cup will stay at 20ºC and the water will stay at 100ºC. An empty paper cup is the same temperature as the air in the room. A student fills the cup with cold water. Which of the following describes how thermal/heat energy is transferred? Support your answer A. Thermal/heat energy is transferred from the cold water to the cup until they are at the same temperature. B. Thermal/heat energy is transferred from the cup to the cold water until they are at the same temperature. C. Thermal/heat energy is transferred from the cup to the cold water until the cup has no more thermal energy. D. Thermal/heat energy is not transferred between the cup and the cold water. A student is holding a cold piece of metal in her hand. While she is holding the piece of metal, her hand gets colder. Does the piece of metal get warmer? Why or why not? Support your answer A. Yes, the piece of metal will get warmer because some thermal energy is transferred from the metal to the student’s hand. B. Yes, the piece of metal will get warmer because some thermal energy is transferred from the student’s hand to the metal. C. No, the piece of metal will stay at the same temperature because an equal amount of thermal energy is exchanged between the student’s hand and the metal. D. No, the piece of metal will stay at the same temperature because thermal energy is not transferred between the student’s hand and the metal.