Investigation 5 Practice `13-Z Write your answers to the following on
advertisement

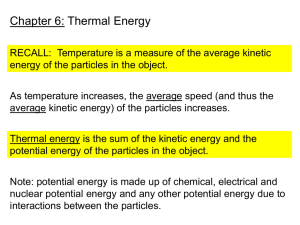
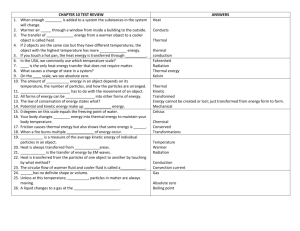
Investigation 5 Practice ’13-Z Write your answers to the following on a separate piece of paper. Bring with you to the retake session you scheduled. 1. Explain how energy transfers between objects of the same temperature 2. Explain how energy transfers between objects of different temperatures 3. Energy “flow” is the transfer of energy from one place to another. Which direction does energy flow? 4. What equation is used to calculate the final temperature when equal masses of water are mixed? 5. What equation is used to calculate how much heat energy (calories) is transferred to or from a mass of water? A plastic bottle filled with warm air is submerged in cold water. 1. What direction does energy flow to cool the air inside the bottle 2. List the materials the energy would have to flow through. 3. How does energy transfer actually happen to cool the air inside the bottle? 4. How long does the energy transfer happen? Write the 3 formulas for finding final temp., change in temp. and calories transferred. Stephen mixed 25 mL of water at 15C and 25 mL of water at 65C. Calculate the answers to the following questions. For each one, list the formula, plug in the numbers (with units) and record the final answer. What is the final temperature of the water? What is the change in temperature of the water? How much heat energy was transferred from the hot water? How much heat energy was transferred to the cold water? When a student mixes 50 mL of water at 15 ºC with 50 mL of water at 80ºC, what happens to the particles? 1. The warm water particles _________________ with the cold water particles 2. The warm water particles start to move _____________ and in the __________________ direction 3. The cold water particles start to move _____________ and in the _______________ direction 4. How much heat energy transferred from the hot water to the cold water (3 steps) Burning a peanut warmed 10 grams of water from 20ºC to 100ºC. How many calories were contained in the peanut? (3 steps) A student has two identical glasses of milk except that the temperature of the milk in one glass is 15 ºC and the temperature of the milk in the other glass is 80ºC. The glass of milk at which temperature has more heat energy? Explain why… Multiple Choice – choose your answer and then SUPPORT with reasoning or evidence why your answer is correct…. The temperature of a plastic cup is 20ºC. It is filled with water that is 10ºC. Which of the following describes how thermal/heat energy is transferred? Support your answer A. Thermal/heat energy is transferred from the water to the cup until they are both at 15ºF. B. Thermal/heat energy is transferred from the cup to the water until they are both at 15ºF. C. Thermal/heat energy is transferred from the cup to the water until the cup is at 10ºC and the water is at 20ºC. D. No thermal/heat energy is transferred between the cup and the water, so the cup will stay at 20ºC and the water will stay at 100ºC. An empty paper cup is the same temperature as the air in the room. A student fills the cup with cold water. Which of the following describes how thermal/heat energy is transferred? Support your answer A. Thermal/heat energy is transferred from the cold water to the cup until they are at the same temperature. B. Thermal/heat energy is transferred from the cup to the cold water until they are at the same temperature. C. Thermal/heat energy is transferred from the cup to the cold water until the cup has no more thermal energy. D. Thermal/heat energy is not transferred between the cup and the cold water. A student is holding a cold piece of metal in her hand. While she is holding the piece of metal, her hand gets colder. Does the piece of metal get warmer? Why or why not? Support your answer A. Yes, the piece of metal will get warmer because some thermal energy is transferred from the metal to the student’s hand. B. Yes, the piece of metal will get warmer because some thermal energy is transferred from the student’s hand to the metal. C. No, the piece of metal will stay at the same temperature because an equal amount of thermal energy is exchanged between the student’s hand and the metal. D. No, the piece of metal will stay at the same temperature because thermal energy is not transferred between the student’s hand and the metal. In the morning, a student puts a warm can of soda in a cooler filled with ice. She closes the cooler. At lunch, the can of soda is colder. What happened to the total amount of energy in the cooler containing the can of soda and the ice? (Assume that no energy is transferred into or out of the cooler when it is closed.) Support your answer A. The total amount of energy increased because the energy that the can of soda transferred to the ice is greater than the energy that the ice lost. B. The total amount of energy decreased because the can of soda has less energy than it started with, and the amount of energy the ice has did not change. C. The total amount of energy stayed the same because the amount of energy that the can of soda lost is equal to the amount of energy that the ice gained. D. The total amount of energy stayed the same because being warmer or colder does not affect the amount of energy objects have.