Chemistry I Chapter 5 Outline THE PERIODIC LAW Atoms of the

Chemistry I Chapter 5 Outline
THE PERIODIC LAW
Atoms of the heavier elements form when stars explode, such as when a supernova causes a nebula.
For graded assessments, you will need to be familiar with the following terms: periodic law, periodic table, lanthanides, actinides, alkali metals, alkalineearth metals, transition elements, main-group elements, halogens, atomic radius, ion, ionization, ionization energy, electron affinity, cation, anion, valence electrons, electronegativity.
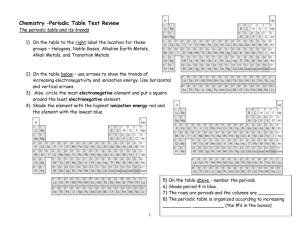
I. HISTORY OF THE PERIODIC TABLE
_____________________ arranged the elements in order of increasing atomic masses. This Russian chemist noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. This repeating pattern is referred to as ____________. An English scientist arranged the elements by increasing ____________ and as a result, the elements in the periodic table _____________________________. This English scientist was ______________. Mendeleev’s principle of periodicity is correctly stated in what is known as ____________________.
The three sets of elements that were added to the modern periodic table after
Mendeleev and Mosely were the _____________, the _____________, and the
______________. Why are the noble gases unreative? _______________________
____________________________________________________________________.
Why were the noble gases undiscovered by Mendeleev and Mosely?
_______________________________________________________________.
(STANDARD CLE 3221.1.2)
II. ELECTRON CONFIGURATION AND THE PERIODIC TABLE
Generally, the electron configuration of an atom’s ________________________ governs the atom’s chemical properties. The length of each period is determined by the
__________________________________ the sublevels being filled in that period.
Which elements do the s block contain – in other words, which two groups.
__________________________ Which groups consist of the d block elements?
__________________________. Which groups consist of the p block elements?
___________________. Which groups consist of the f block elements?
_________________________. Give three properties of the metals that are found in the s block. _______________________________________________________ Because of alkali metals’ extreme reactivity, they must be stored in ______________. Compared to alkali metals, alkaline earth metals are __________________, _______________ &
________________. Of groups 1 and 2 metals, which has a higher melting point?
_____________. Which element is unique and has properties that do not resemble those of any other group? _______________. Some deviations from expected, orderly d sublevel filling occur in which groups? ___________ What are some of the properties of transition elements? ___________________________________________________
__________________________________________. The p-group elements are also called _________________ and they make up groups _______________. Electrons add to a ___________ only after the s sublevel in the same energy level is filled. The
___________ are the most reactive nonmetals. They react vigorously with metals to form __________. Why are they so reactive? ________________________________
List some properties of the p block elements __________________________________
_____________________________________________________________________.
(STANDARD CLE 3221.MATH.2)
III. ELECTRON CONFIGURATION AND PERIODIC PROPERTIES
The size of an atom is defined by the edge of its _______________. Why do atomic radii decrease across periods and increase down groups?
______________________________________________________________________
_______________________. Ionization energies are highest for those atoms that have a _________________. Electron affinity is a measure of how much energy is required
to add an ____________ to a neutral atom. The formation of a cation by the loss of one or more electrons always leads to a __________ in atomic radius. This is because the removal of the highest-energy-level electrons results in a ______________. The formation of an anion by the gain of one or more electrons always leads to a
_____________ in atomic radius. This is because the total positive charge of the nucleus remains unchanged when an electron is added to an __________ or an
__________. Valence electrons are often located in ________________ main-energy levels. For example, elements of group 1 always have ______ (how many) valence electrons. _____________ hold atoms together in chemical compounds. The most electronegative element is __________. The properties f the d block elements (all metals) vary less and with less regularity that those of the ______________________.
HOW….
ATOMIC RADII
IONIZATION ENERGIES
PERIOD TRENDS GROUP TRENDS
Decrease from left to right Increase from top to bottom
Increase from left to right Decrease from top to bottom
ELECTRONEGATIVITY Generally increase across a period
Decrease down a group or remain about the same
Please be familiar with the definitions and the concepts that are associated with the terms that are listed in the above table. These periodic trends are very important and will be seen again on your EOC.
(STANDARD CLE 3221.INQ.3)