The Bohr model EXPLAINED
advertisement

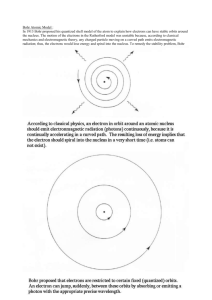
The Bohr Model…explained The Bohr model consists of four principles: 1) Electrons assume only certain orbits around the nucleus. These orbits are stable and called "stationary" orbits. 2) Each orbit has an energy associated with it. For example the orbit closest to the nucleus has an energy E1, the next closest E2 and so on. 3) Light is emitted when an electron jumps from a higher orbit to a lower orbit and absorbed when it jumps from a lower to higher orbit. 4) The energy and frequency of light emitted or absorbed is given by the difference between the two orbit energies, e.g., E(light) = Ef - Ei = E(light)/h h= Planck's constant = 6.627x10-34 Js where "f" and "i" represent final and initial orbits. The Planck Hypothesis In order to explain the frequency distribution of radiation from a hot cavity (blackbody radiation) Planck proposed the ad hoc assumption that the radiant energy could exist only in discrete quanta which were proportional to the frequency. This would imply that higher modes would be less populated and avoid the ultraviolet catastrophe of the Rayleigh-Jeans Law. The quantum idea was soon seized to explain the photoelectric effect, became part of the Bohr theory of discrete atomic spectra, and quickly became part of the foundation of modern quantum theory. With these conditions Bohr was able to explain the stability of atoms as well as the emission spectrum of hydrogen. According to Bohr's model only certain orbits were allowed which means only certain energies are possible. These energies naturally lead to the explanation of the hydrogen atom spectrum: Bohr's model was so successful that he immediately received world-wide fame. Unfortunately, Bohr's model worked only for hydrogen. Thus the final atomic model was yet to be developed. Failures of the Bohr Model While the Bohr model was a major step toward understanding the quantum theory of the atom, it is not in fact a correct description of the nature of electron orbits. Some of the shortcomings of the model are: 1. It fails to provide any understanding of why certain spectral lines are brighter than others. There is no mechanism for the calculation of transition probabilities. 2. The Bohr model treats the electron as if it were a miniature planet, with definite radius and momentum. This is in direct violation of the uncertainty principle which dictates that position and momentum cannot be simultaneously determined. The Bohr model gives us a basic conceptual model of electrons orbits and energies. The precise details of spectra and charge distribution must be left to quantum mechanical calculations, as with the Schrodinger equation. Problems with the Bohr Model It violates the Heisenberg Uncertainty Principle because it considers electrons to have both a known radius and orbit. The Bohr Model provides an incorrect value for the ground state orbital angular momentum. It makes poor predictions regarding the spectra of larger atoms. It does not predict the relative intensities of spectral lines. The Bohr Model does not explain fine structure and hyperfine structure in spectral lines. It does not explain the Zeeman Effect. http://www.colorado.edu/physics/2000/quantumzone/bohr.html interactive demo http://www.upscale.utoronto.ca/PVB/Harrison/BohrModel/Flash/BohrModel.html interactive demo http://www.youtube.com/watch?v=R7OKPaKr5QM video