Yr 9 Atoms & Ions Curriculum Overview

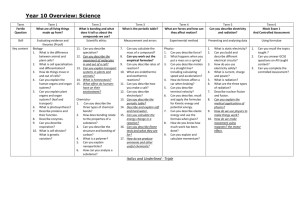
UNIT- Atoms & Ions
Introduction
Students will build on chemical knowledge from previous years, to bring them up to a skills and knowledge level sufficient for entry to VCE sciences.
In this unit students will:
Review their understanding of the Particle Model and basic Atomic
Theory
Provide an understanding of the various theories to explain atoms and their structure
Explain the formation of ions and ionic compounds
Review the Periodic Table and its role in organising the elements
Become familiar with the role of key scientists in the development of the periodic table, and be aware that theories change over time.
Extend familiarity with chemical symbols and formulae, including expressing chemical changes using symbols
Use language of Chemistry competently
Conduct a series of practical activities that demonstrate common chemical reactions.
Be able to write full balance chemical equations.
Become familiar with the basic models for metallic, ionic and covalent bonding.
Key Competencies
KEY KNOWLEDGE
The Key Knowledge that students will gain from this unit include the:
structure of an atom including electron configuration, and isotopes
arrangement of elements into the periodic table, including the significance of groups and periods.
Use the periodic table to determine atomic number, relative atomic mass, number of subatomic particles (protons, neutrons and electrons)
symbols/formulae of designated elements/compounds
recognition of signs that a chemical reaction has occurred
Classification of chemical reactions.
writing balanced chemical equations using words, symbols,
KEY SKILLS
The Key Skills that students will improve during the completion of this unit are:
Defining and using designated chemical terminology appropriately.
Using the theory learnt in the unit to solve problems.
Conducting practical investigations.
Recording and interpreting experimental results to make connections to the key knowledge.
Evaluating practical investigations to determine possible sources of experimental error and identify possible alterations to the experimental design.
Time management and meeting deadlines.
KEY TERMINOLOGY
The Key Terminology that students will become familiar with, use appropriately and spell correctly during this unit includes:
KEY TERMINOLOGY I
Element
Electron
Cation
Products
Nucleus
Periodic table
Neutron
Anion
Reactants
Precipitate
Shell
Compound
Metal
Atomic mass
Molecule
Chemical formulae
Atom
Proton
Non metal
Atomic number
Isotope
Chemical change
UNIT RESOURCES AVAILABLE
Science Dimensions 3- Chapter 1 & 2
Science Links 3- Chapter 2 & 3
Video- World of Chemistry ‘Bonding’
Video- Electron arrangement and bonding
POSSIBLE ASSESSMENT TASKS INCLUDE
1.
Topic Test
2.
Practical report- eg. Precipitation reactions
3.
Electron Shell diagram worksheets