SUPPLEMENTARY METHODS Immunosuppressive regimens All
advertisement

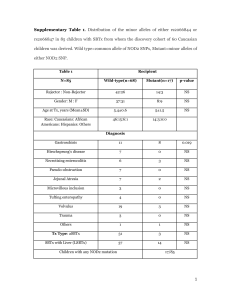
SUPPLEMENTARY METHODS Immunosuppressive regimens All patients received induction therapy consisting of rabbit antithymocyte globulin or basiliximab. Target blood levels of maintenance immunosuppressive therapies were : i) Ciclosporin: blood through level (T0) = 120 ng/mL, ii) Tacrolimus blood through level (T0) between 7 and 8 ng/mL; iii) Mycophenolic acid: AUC0-12 between 30 and 60 µg/h/mL; iiii) Everolimus: blood through level (T0) between 4 and 8 ng/mL iiiii) for patients maintained on corticosteroids, a daily dose is of 0.1 to 0.2 mg/kg body weight. Supplementary Table 1: Description of the time frame of index biopsy in the entire cohort (test + validation cohorts) Normal allografts before 1 year Normal allografts after 1 year Rejecting allografts before 1 year Rejecting allografts after 1 year Total Test cohort 10 20 9 21 60 Validation cohort 10 12 16 15 53 Test + validation 20 32 25 36 113 Footnote: the cohort, is including rejection episodes occurring in the first year post transplant (n=25/61, 41%) as well as rejection occurring after 1 year post transplant (n=36/61, 59%). In our cohort, among the 36 late rejection episodes, 12 (33%) are T-cell mediated rejection and 24 (67%) are antibody-mediated rejection. Supplementary Table 2: Tissue distribution, known implications, regulations and interactions of the fourteen miRNAs of interest (25, 27 , 28 , 29 , 30, 31, 32 , 33 , 34- 40). Known implications in heart/transplantation - related diseases Known regulations/interactions miRNAs mostly found in the endothelium miR-92a Apoptosis eNOS, JUN/FOS pathway, integrin α5, KLF2/4 miR-221 Hypertrophy, endothelial dysfunction ICAM1, p27, c-kit, Ets-1 miR-296 Angiogenesis, hypertension, ischemia VEGFR2 miR-126 Endothelial damage and injury, lipid VCAM1, SPRED1, PIK3RS, CXCL12. metabolism, angiogenesis Reduced in coronary heart disease. miRNAs involved in inflammatory response miR-155 Cellular migration, cell survival (cytokines), eNOS, RIP1, Ang2, Bcl6, Ets-1, HO-1. necrosis, NK cells. Increased in coronary artery disease miR-142-3p Inflammatory response miR-451 Cell migration Pi3K, ROS, Ago2, AMPK miR-182 Present in PBMC Increased in kidney transplanted patients with AR miR-10a Atherosclerosis, shear stress MAPK, NFκB Increased in kidney transplanted patients with AR miR-181b Vascular inflammation, atherosclerosis NFκB (importin α3), TGFβ miR-181a Acute cellular rejection, endothelial Increased in CD4 cells senescence Cardiac-enriched miRNAs miR-21 Ischemia, endothelial cell proliferation, Spry1, ERK/MAPK, PI3K/AKT, eNOS, pro-fibrosis, pro-apoptosis, hypertension, BCL2/PTEN, Smad family, PPARα shear stress miR-31 Fibrosis HIF-1, IL-2, e-selectin miR-208a Infarct, hypertrophy α/β myosin, connexins Supplementary Table 3: Grades of heart allograft rejection using ISHLT classifications in the test and validation cohorts NORMAL ALLOGRAFTS (n,%) TEST VALIDATION COHORT COHORT (n=60) (n=53) p 30 (50%) 22 (41.5%) 0.4 REJECTING GRAFTS (n,%) 30 (50%) 31 (58.5%) 0.4 T-cell mediated rejection (n,%) 11 (18.3%) 14 (26.4%) 0.4 1R 3 (5%) 2 (3.8%) 2R 6 (10%) 9 (14%) 3R 2 (3.3%) 3 (5.7%) 19 (31.7%) 17 (32.1%) pAMR1-I+ 2 (3.3%) 2 (3.8%) pAMR1-H+ 7 (11.7%) 4 (7.6%) pAMR2 10 (16.7%) 11 (20.7%) pAMR3 0 (0%) 0 (0%) 0R and pAMR0 Antibody-mediated rejection (n,%) 1.0 Supplementary Table 4: Patient characteristics of the validation cohort Recipient age, median (median, IQR [25 - 75]) NORMAL REJECTING ALLOGRAFTS ALLOGRAFTS (n=22) (n=31) P value 27 [11 - 63] 39 [24 - 49] 0.9 17 (77%) 17 (55%) 0.1 1 (4%) 2 (6%) 1.0 Non-ischemic cardiopathy 15 (68%) 17 (55%) 0.4 Ischemic cardiopathy 3 (14%) 9 (29 %) 0.3 Valvular cardiomyopathy 0 (0%) 0 (0%) 1.0 Recipient gender, male, n (%) Primary heart disease, n (%) Congenital cardiopathy Retransplant 0 (0%) 1 (3%) 1.0 3 (14 %) 2 (6%) 0.6 27 [18 - 43] 39 [29 - 48] 0.1 15 (68 %) 19 (61%) 0.8 208 [160 - 245] 218 [176 - 266] 0.7 Steroids 17 (77%) 26 (84%) 0.7 Calcineurin inhibitors 21 (95%) 30 (97%) 1.0 Mycofenolate acid 19 (86%) 28 (90%) 0.7 m-TOR inhibitors 4 (18%) 3 (10%) 0.4 Azathioprine 3 (14%) 0 (0%) 0.07 23 [3 - 44] 19 [11 - 33] 0.99 Miscellaneous Donor age, median (median, IQR [25 - 75]) Donor gender, male, n (%) Cold ischemic time, median (median, IQR [25 - 75]) Maintenance Immunosuppression (%) Time between Tx and Index EMB, Months IQR [25 - 75] Supplementary Figure 1: Negative control of In situ hybridization using scrambled probe control Supplementary Figure 2: Dendrogram and unsupervised principal component analysis. A: Individual overview of the tissular expression of the 14 miRNAs of interest (Figure 1A). Each variable(miRNA) in an individual patient is colored according to the threshold for each miRNA graded from 0 to 3 according to quartiles. B: Unsupervised principal component analysis (PCA). The PCA analysis examines the 60 heart transplant patients using the 14 miRNAs of interest according to the heart allograft status: normal, Tcell mediated rejection and antibody-mediated rejection Supplementary Figure 3: Sensitivity analysis: Diagnostic accuracy of miRNAs for predicting T-cell mediated rejection (all cases from the test and the validation set) Supplementary Figure 4: Sensitivity analysis: Diagnostic accuracy of miRNAs for predicting antibody-mediated rejection (all cases from the test and the validation set) Supplementary Figure 5: Sensitivity analysis: A: miRNA expression according to time post transplantation. B: Diagnostic accuracy according to time post transplantation A footnote: ****: p<0.0001 B