October 19, 2012
advertisement
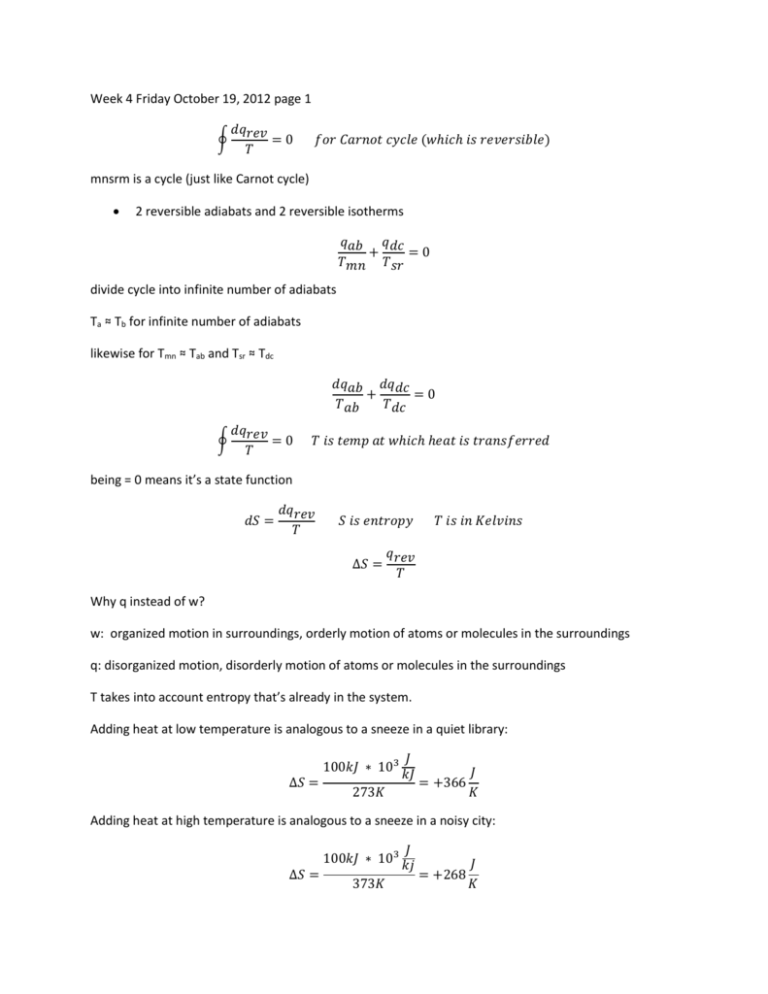
Week 4 Friday October 19, 2012 page 1 ∮ 𝑑𝑞𝑟𝑒𝑣 =0 𝑇 𝑓𝑜𝑟 𝐶𝑎𝑟𝑛𝑜𝑡 𝑐𝑦𝑐𝑙𝑒 (𝑤ℎ𝑖𝑐ℎ 𝑖𝑠 𝑟𝑒𝑣𝑒𝑟𝑠𝑖𝑏𝑙𝑒) mnsrm is a cycle (just like Carnot cycle) 2 reversible adiabats and 2 reversible isotherms 𝑞𝑎𝑏 𝑞𝑑𝑐 + =0 𝑇𝑚𝑛 𝑇𝑠𝑟 divide cycle into infinite number of adiabats Ta ≈ Tb for infinite number of adiabats likewise for Tmn ≈ Tab and Tsr ≈ Tdc 𝑑𝑞𝑎𝑏 𝑑𝑞𝑑𝑐 + =0 𝑇𝑎𝑏 𝑇𝑑𝑐 ∮ 𝑑𝑞𝑟𝑒𝑣 =0 𝑇 𝑇 𝑖𝑠 𝑡𝑒𝑚𝑝 𝑎𝑡 𝑤ℎ𝑖𝑐ℎ ℎ𝑒𝑎𝑡 𝑖𝑠 𝑡𝑟𝑎𝑛𝑠𝑓𝑒𝑟𝑟𝑒𝑑 being = 0 means it’s a state function 𝑑𝑆 = 𝑑𝑞𝑟𝑒𝑣 𝑇 𝑆 𝑖𝑠 𝑒𝑛𝑡𝑟𝑜𝑝𝑦 𝑇 𝑖𝑠 𝑖𝑛 𝐾𝑒𝑙𝑣𝑖𝑛𝑠 𝑞 ∆𝑆 = 𝑟𝑒𝑣 𝑇 Why q instead of w? w: organized motion in surroundings, orderly motion of atoms or molecules in the surroundings q: disorganized motion, disorderly motion of atoms or molecules in the surroundings T takes into account entropy that’s already in the system. Adding heat at low temperature is analogous to a sneeze in a quiet library: 100𝑘𝐽 ∗ 103 ∆𝑆 = 𝐽 𝑘𝐽 273𝐾 = +366 𝐽 𝐾 Adding heat at high temperature is analogous to a sneeze in a noisy city: 100𝑘𝐽 ∗ 103 ∆𝑆 = 373𝐾 𝐽 𝑘𝑗 = +268 𝐽 𝐾 2 ∆𝑆 = 𝑆2 − 𝑆1 = ∫ 1 𝑑𝑞𝑟𝑒𝑣 𝑇 𝑐𝑙𝑜𝑠𝑒𝑑 𝑠𝑦𝑠𝑡𝑒𝑚, 𝑟𝑒𝑣𝑒𝑟𝑠𝑖𝑏𝑙𝑒 𝑝𝑟𝑜𝑐𝑒𝑠𝑠 Any isolated system is closed so it applies to an isolated system too. ∆Sirrev = ∆Srev note: S is an extensive state function prove: partitioned container 𝑙𝑒𝑓𝑡 𝑝𝑎𝑟𝑡𝑖𝑡𝑖𝑜𝑛: 𝑑𝑆1 = 𝑑𝑞1 𝑇 𝑟𝑖𝑔ℎ𝑡 𝑝𝑎𝑟𝑡𝑖𝑡𝑖𝑜𝑛: 𝑑𝑆2 = 𝑑𝑞2 𝑇 dS = dS1 + dS2 ∆S = ∆S1 + ∆S2 S = S1 + S2 so S is extensive 𝑆 𝐽 𝑐𝑎𝑙 𝑢𝑛𝑖𝑡𝑠: 𝑆𝑚 = 𝑖𝑛 𝑜𝑟 𝑛 𝑚𝑜𝑙 𝐾 𝑚𝑜𝑙 𝐾 𝑆 𝑖𝑛 𝐽 𝑐𝑎𝑙 𝑜𝑟 𝐾 𝐾 Calculation of entropy changes: 𝑑𝑆 = 𝑑𝑞𝑟𝑒𝑣 𝑇 2 ∆𝑆 = ∫ 𝑑𝑞𝑟𝑒𝑣 1 𝑇 ∆𝑆𝑟𝑒𝑣 = ∆𝑆𝑖𝑟𝑟𝑒𝑣 1. Cyclic process ∆S=0 System (not surroundings) is always implied 2. rev adiabatic process dqrev=0 so ∆S=0 3. rev phase change at constant T,P 2 𝑑𝑞𝑟𝑒𝑣 a. ∆𝑆 = ∫1 𝑇 1 = ∫ 𝑑𝑞𝑟𝑒𝑣 = 𝑇 𝑞𝑟𝑒𝑣 𝑇 = 𝑞𝑃 𝑇 = ∆𝐻 𝑇 b. ∆H is latent heat of transition c. If ∆H>0 then ∆S>0. If ∆H<0 then ∆S<0. 4. rev isothermal process a. T is constant 1 b. ∆𝑆 = 𝑇 ∫ 𝑑𝑞𝑟𝑒𝑣 = c. ∆𝑆 = 𝑞𝑟𝑒𝑣 𝑇 𝑞𝑟𝑒𝑣 𝑇 𝑆 𝑖𝑠 𝑛𝑜𝑡 𝑣𝑒𝑟𝑦 𝑝𝑟𝑒𝑠𝑠𝑢𝑟𝑒 𝑠𝑒𝑛𝑠𝑖𝑡𝑖𝑣𝑒 5. rev change of state of a perfect gas (V1T1 → V2T2) a. dqrev=dU-dwrev 1st law b. 𝑑𝑞𝑟𝑒𝑣 = 𝐶𝑉𝑑𝑇 + 𝑃𝑑𝑉 = 𝐶𝑉𝑑𝑇 + c. 𝑑𝑞𝑟𝑒𝑣 𝑇 = 𝐶𝑉𝑑𝑇 𝑇 + 𝑛𝑅 𝑑𝑉 𝑉 𝑛𝑅𝑇 𝑑𝑉 𝑉 𝑡ℎ𝑒𝑛 𝑖𝑛𝑡𝑒𝑔𝑟𝑎𝑡𝑒 𝑡ℎ𝑒𝑛 𝑑𝑖𝑣𝑖𝑑𝑒 𝑏𝑦 𝑇 d. ∫ 𝑑𝑞𝑟𝑒𝑣 𝑇 e. ∆𝑆 = f. 𝐶𝑉(𝑇)𝑑𝑇 𝑛𝑅 + ∫ 𝑉 𝑑𝑉 𝑇 2 𝐶 (𝑇) 𝑉 ∫1 𝑉𝑇 𝑑𝑇 + 𝑛𝑅𝑙𝑛 𝑉2 1 =∫ Now assume CV is constant over temperature range: 𝑇 𝑉 g. ∆𝑆 ≈ 𝐶𝑉𝑙𝑛 𝑇2 + 𝑛𝑅𝑙𝑛 𝑉2 1 1 h. So expansion increases entropy and heating increases entropy. i. ∆S↑ when T↑ or when V↑ 6. irrev change of state of a perfect gas a. ∆Sirrev = ∆Srev but qrev ≠ qirrev since T plays role 7. constant pressure heating (no phase change) a. dqrev=dqP=CPdT=TdS b. 𝑑𝑆 = c. ∆𝑆 = 𝑑𝑞𝑟𝑒𝑣 𝐶 𝑑𝑇 = 𝑃𝑇 𝑇 𝑇 𝐶 (𝑇) ∫𝑇 2 𝑃𝑇 𝑑𝑇 1 d. For constant CP: 𝑇 𝑑𝑇 𝑇 = 𝐶𝑃𝑙𝑛 𝑇2 𝑇 1 1 𝑇 𝐶𝑃𝑙𝑛 𝑇2 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 𝑃 𝑎𝑛𝑑 1 e. ∆𝑆 = 𝐶𝑃 ∫𝑇 2 f. ∆𝑆 = 𝐶𝑃, 𝑛𝑜 𝑝ℎ𝑎𝑠𝑒 𝑐ℎ𝑎𝑛𝑔𝑒, 𝑟𝑒𝑣 𝑜𝑟 𝑖𝑟𝑟𝑒𝑣 8. general change of state (P1T1 → P2T2) a. ∆S=? wait until chapter 4 b. For problem 31, use PV=nRT 9. irreversible phase change a. We want to go from liquid H2O at -10˚C and 1atm straight to ice at -10˚C and 1 atm, which is irreversible. b. Instead, we’ll add two intermediate steps: i. liquid H2O at 0˚C and 1 atm ii. solid H2O at 0˚C and 1 atm c. ∆Srev is the reversible step from liquid H2O at -10˚C and 1atm to liquid H2O at 0˚C and 1 atm. d. ∆S’rev is the reversible step from liquid H2O at 0˚C and 1 atm to solid H2O at 0˚C and 1 atm. e. ∆S”rev is the reversible step from solid H2O at 0˚C and 1 atm to ice at -10˚C and 1 atm. f. Since S is extensive: ∆S=∆Srev + ∆S’rev + ∆S”rev 𝑇 g. ∆𝑆𝑟𝑒𝑣 = 𝐶𝑃𝑙𝑛 𝑇2 1 𝐶𝑃 𝑓𝑜𝑟 𝑙𝑖𝑞𝑢𝑖𝑑 𝑤𝑎𝑡𝑒𝑟 h. ∆𝑆′𝑟𝑒𝑣 = = i. ∆𝑆"𝑟𝑒𝑣 ∆𝐻𝑓𝑟𝑒𝑒𝑧𝑖𝑛𝑔 𝑇 𝑇 = 𝐶𝑃𝑙𝑛 2 𝑇1 −∆𝐻𝑚𝑒𝑙𝑡𝑖𝑛𝑔 𝑇 𝐶𝑃 𝑓𝑜𝑟 𝑠𝑜𝑙𝑖𝑑 𝑤𝑎𝑡𝑒𝑟







