click here for slo review!
advertisement
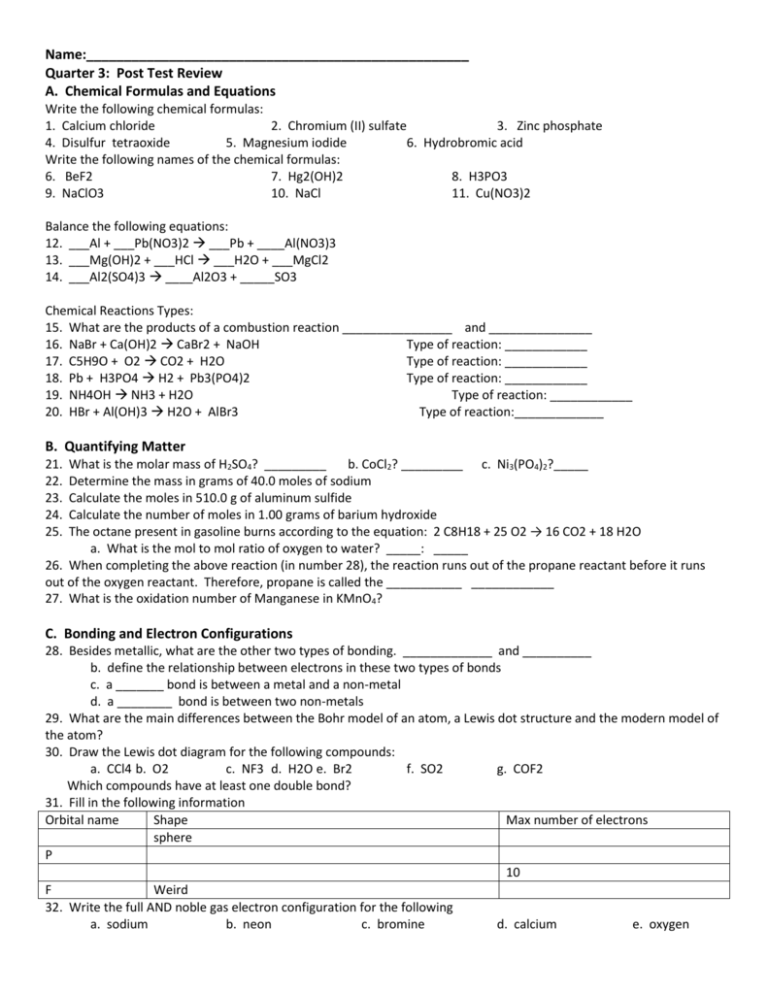
Name:___________________________________________________ Quarter 3: Post Test Review A. Chemical Formulas and Equations Write the following chemical formulas: 1. Calcium chloride 2. Chromium (II) sulfate 3. Zinc phosphate 4. Disulfur tetraoxide 5. Magnesium iodide 6. Hydrobromic acid Write the following names of the chemical formulas: 6. BeF2 7. Hg2(OH)2 8. H3PO3 9. NaClO3 10. NaCl 11. Cu(NO3)2 Balance the following equations: 12. ___Al + ___Pb(NO3)2 ___Pb + ____Al(NO3)3 13. ___Mg(OH)2 + ___HCl ___H2O + ___MgCl2 14. ___Al2(SO4)3 ____Al2O3 + _____SO3 Chemical Reactions Types: 15. What are the products of a combustion reaction ________________ and _______________ 16. NaBr + Ca(OH)2 CaBr2 + NaOH Type of reaction: ____________ 17. C5H9O + O2 CO2 + H2O Type of reaction: ____________ 18. Pb + H3PO4 H2 + Pb3(PO4)2 Type of reaction: ____________ 19. NH4OH NH3 + H2O Type of reaction: ____________ 20. HBr + Al(OH)3 H2O + AlBr3 Type of reaction:_____________ B. Quantifying Matter 21. 22. 23. 24. 25. What is the molar mass of H2SO4? _________ b. CoCl2? _________ c. Ni3(PO4)2?_____ Determine the mass in grams of 40.0 moles of sodium Calculate the moles in 510.0 g of aluminum sulfide Calculate the number of moles in 1.00 grams of barium hydroxide The octane present in gasoline burns according to the equation: 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O a. What is the mol to mol ratio of oxygen to water? _____: _____ 26. When completing the above reaction (in number 28), the reaction runs out of the propane reactant before it runs out of the oxygen reactant. Therefore, propane is called the ___________ ____________ 27. What is the oxidation number of Manganese in KMnO4? C. Bonding and Electron Configurations 28. Besides metallic, what are the other two types of bonding. _____________ and __________ b. define the relationship between electrons in these two types of bonds c. a _______ bond is between a metal and a non-metal d. a ________ bond is between two non-metals 29. What are the main differences between the Bohr model of an atom, a Lewis dot structure and the modern model of the atom? 30. Draw the Lewis dot diagram for the following compounds: a. CCl4 b. O2 c. NF3 d. H2O e. Br2 f. SO2 g. COF2 Which compounds have at least one double bond? 31. Fill in the following information Orbital name Shape Max number of electrons sphere P 10 F Weird 32. Write the full AND noble gas electron configuration for the following a. sodium b. neon c. bromine d. calcium e. oxygen 33. How many more electrons do the following need to satisfy the octet rule? a. [Ne] 3s23p3 b. 1s2,2s2, 2p4 D. Gas Laws 34. What is the kinetic molecular theory? 35. What is Boyle’s law? _______________________________ b. What is the relationship between pressure and volume? 36. What is Charles’s law? _______________________________ b. What is the relationship between volume and temperature? 37. What is the ideal gas law? ______________________________ 38. When using the ideal gas law what are the units for the following? Temperature = Pressure = Volume = n= r= 39. What is STP? 40. ________ mmHg = __________torr = _________atm = __________kilopascals Convert 2983.4 mmHg into torr. ___________________ convert in Pascals ________________ 41. The volume of a balloon is 300. mL and has a pressure of 1.5 atm. If the pressure is increased to 3.0 atm, what is the new volume? 42. What is the volume of a 2.0 mol sample of gas at 800 mm Hg and 5.0 0 C? E. Acids and Bases 43. 44. 45. 46. 47. Define acid and base. How is water both an acid and a base? What is the expression for pOH? pH? In the reaction, HCl + H2O ↔ H3O+ + Cl-, what are the conjugate base pairs? Discuss each diagram in terms of precision and accuracy.