C.M.Muilwijk, non-invasive endocrine monitoring techniques in the

Non-invasive endocrine monitoring in the aardwolf
(Proteles cristatus) using faecal samples
Drs. C.M. Muilwijk
Faculty of Veterinary Science, Utrecht University, the Netherlands 2011
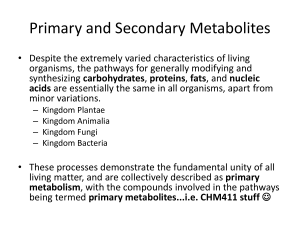
Four aardwolves (two males and two females) in South Africa were studied to indentify a suitable test system for assessing gonadal and adrenocortical endocrine function based on faecal analysis during four months. It was possible to determine the following classes of hormones in the faeces of aardwolves: androgen, progestagen, estrogen and glucocorticoid metabolites. For androgen and progestagen metabolites a significant difference between the females and males was measured. For a physiological validation of faecal glucocorticoid metabolites, two aardwolves were darted and injected with exogenous ACTH. The GCM levels in the feaces increased significantly within respectively 3-20 hours after the injection. This ACTH challenge showed that the measured feacal glucocorticoid metabolites were relevant indicators of adrenal activity in aardwolves. It can therefore be useful as a non-invasive tool for measuring a physiological stress response non-invasively in this species.
INTRODUCTION
Although the current IUCN status of the aardwolf ( Proteles cristatus ) is
“Least Concerned” [IUCN 2008], there is a general lack of biological knowledge of these myrmecophagous mammals. More information of the reproductive endocrinology and their behavior ecology can be helpful to protect this species and the vitality of their ecosystems.
For a successful investigation of reproductive-endocrine relationships in the aardwolf, collection of repeated samples for hormonal evaluations is necessary.
[Schwarzenberger et al., 1996]. However, collecting blood from free-living aardwolves requires anesthesia, which is usually stressful and dangerous for the animal and also expensive and impractical.
Furthermore, physiological stress by darting animals may obscure the normal hormonal milieu. An alternative option is using non-invasive sampling methods; urinary, salivary, sweat, feathers, milk, nails, hair and faecal sampling [Cook et al,
2000], which reduce pain and suffering for the animals. Additionally, non-invasive materials can be collected even daily for longitudinal studies [Monfortet al., 2002;
Dloniak et al., 2003]. An extra benefit of collecting faeces instead of blood is that a pooled fraction of plasma hormones is measured and not the fluctuations of hormones in the blood. [Creel et al., 1996;
Goymann et al., 1999].
However, noninvasive measurements need to be validated to ensure that the measured compounds reflect circulating levels of the biologically active target agents [Cekan,
1975; Schwarzenberger et al, 1996].
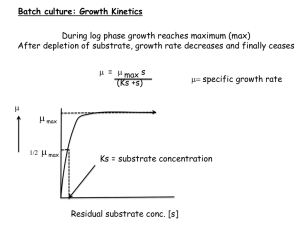
Alongside measuring the reproductive hormones, it is also interesting to learn more about the physiological responses to environmental and social stressors. Adrenal glucocorticoids play an important role in the homeostasis of an individual. If the homeostasis of an animal is disturbed by physiological or behavioral stressors, the animal responses with the secretion of glucocorticoids by the hypothalamicpituitary-adrenal axis (HPA axis)
[Ganswindt et al., 2010]. Because it is impossible to predictably find wild animals with elevated stress levels, the HPA axis is triggered with a conducted adrenocorticotrophic hormone (ACTH). To measure whether the glucocorticoid levels increase in the aardwolf after the ACTH injection, faecal and blood samples were collected both before and after the injection.
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples
In recent years, reliable noninvasive methods have been established for assessing hormone metabolite levels in faeces for a large number of mammalian species [Schwarzenberger et al., 1996;
Palme-Möstl, 1997; Ganswindt et al.,
2003]. One of the species for which this has been examined is the spotted hyena
( Crocuta crocuta ), a close relative of the aardwolf [Goymann et al., 1999; Dloniak et al., 2004]. Because of the lack of knowledge concerning the hormone metabolite levels of aardwolves and the proven usefulness of using faeces for assessing the hormone metabolite levels in relative species this method was chosen to be applied to aardwolves. Although there were some concerns whether this method would work with this species. The aim of this study was to investigate the hormone metabolite levels of aardwolves by making use of their faeces. The endocrine parameters used in this research contain the following hormone metabolites: androgen, estrogen, progestagen and glucocorticoid. Androgen (testosterone), estrogen and progestagen (gestagen) metabolites were compared between females and males for validation.
Glucocorticoid metabolites were measured in the faeces and blood for the ACTHchallenge. These hormones will give more information about the various aspects of the endocrine activity of this species.
MATERIALS
Study site
This study was conducted between
November 2009 and March 2010 on
Benfontein Game Farm (BGF; 28°50′ S,
24°50′ E), near Kimberley in central South
Africa. Benfontein is 11.000 hectare and is located in an area where Nama Karoo, grassland and Kalahari Savanna meet. The mean annual rainfall is 430 ± 127 mm.
There is a distinct cold and dry period during winter months (–8 to 25 °C), and a hot and rainy period during summer months (8 to 40 °C) [Stenkewitz and
Kamler, 2008].
Study animals
For this study four radio-collared aardwolves ( Proteles cristatus ), two males
(BWM08002, BWM09008) and two females (BWF08006, BWF09009), were used. Together with the spotted, brown, and striped hyena, the aardwolf belongs to the Hyaenidae, the second smallest family of the Carnivora. Aardwolves have an average body mass of 8 – 12 kilogram.
They are social monogamous but sexually polygamous, primarily nocturnal, and obligate termitivores feeding primarily on harvester termites [Williams et al., 1997;
Taylor-Skinner, 2000].
Figure 1. BWM09008, one of the studied
Aardwolves.
The aardwolf is limited to Africa and occurs in two discrete populations. The southern population ranges over most of southern Africa, extending just into southern Angola, southern Zambia, and southwestern Mozambique [Koehler-
Richardson, 1990]. The northern population extends from central Tanzania to northeastern Uganda and Somalia, then narrowly along the coast of Ethiopia and
Sudan to extreme southeastern Egypt
[Koehler-Richardson, 1990]. Our studied animals belong to the southern population.
METHODS
Faecal samples
2 C.M. Muilwijk, Utrecht University
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples
During the day all the radio collared (Sirtrack®, Havelock North, approx. weight 60g/collar) aardwolves were tracked in their dens using VHFtransmitters. Almost every night at least one aardwolf was followed by car. The studied animals were not shy of the motor vehicle and appeared undisturbed when followed within 15 m.
In order to monitor faecal hormonal metabolites, the aardwolf was followed until it defecated. Bi-weekly faecal samples were collected (n = 66). Before these animals defecated, they dug a narrow trench with alternating strokes of the forepaws. Then the aardwolf turned around and squatted over the trench. The first defecation of the evening may be up to 8% of the body mass of the animal [Koehler-
Richardson, 1990]. The faecal samples collected in this study were for the greater part the first defecations of the evening.
The subsequent two or three defecations are much smaller [Koehler-Richardson,
1990]. After defecating, the aardwolf filled the hole with sand and usually deposited a few scent marks before leaving. After the animal had defecated and moved away, the faecal sample was excavated using disposable rubber gloves. A sample was taken from the middle of the bolus to avoid cross-contamination with urine or contamination with other faecal samples in the area. Subsequently, the sample was transferred into a glass vial, immediately placed on ice and was frozen at -20ºC until extracted.
To measure whether the glucocorticoid levels in the faeces increase after the ACTH injection, faecal samples were collected 48 hours before and 48 hours after the injection. The faeces collected in the pre-injection period provided a measure of baseline glucocorticoid levels.
Two aardwolves, BWF08006
(female) and BWM09008 (male), were darted using a CO
2
powered dart gun
(Telinject, Germany) with 36 milligram ketamine hydrochloride (Anaket-V®) per kilogram bodyweight and 0,6 milligram medetomidine hydrochloride (Domitor®).
Approximately 60 minutes after darting the medetomidine was reversed with 3.0 mg atipamezole (Antisedan®).
Both aardwolves were also injected with procaine penicillin and benzathine penicillin (Duplocillin®) at 15 IU per kilogram bodyweight to avoid infections from the dart wounds.
Figure 2. Frozen glass vials with faecal samples.
ACTH challenge
3
Figure 3. One of the studied aardwolves
(BWF08006) was injected intramuscularly with 10
IU of synthetic ACTH.
First blood was taken from the anesthetized aardwolves. The next injection was 10 IU of synthetic adrenocorticotrophic hormone
(Synacthen® depot, Novartis, Australia) intramuscularly. After 60 minutes another blood sample was taken. Blood samples
C.M. Muilwijk, Utrecht University
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples were taken to compare the immunoreactive cortisol in blood with the glucocorticoid metabolites concentrations measured in the faecal samples collected both before and after the ACTH injection for validation.
For the validation of the reproductive hormones bi-weekly faecal samples were collected of each aardwolf, in order to improve the knowledge of the hormone metabolite levels in aardwolves.
Two females and two males were studied to compare the reproductive hormone metabolites between the different sexes.
Extraction and assay
Faecal samples were lyophilized, pulverized and sifted using a mesh strainer to remove fibrous material [Fiess et al.,
1999]. 0,2 g of the faecal powder was then extracted with 3 ml 80% ethanol in water.
After vortexing for 15 minutes the mixture was centrifuged for 10 minutes at 1,500 g.
The supernatant was transferred to a micro-centrifuge tube for hormone analysis. However, due to a highly variable amount of mineral content in the collected samples (49,5-92,5% of total dry weight), the organic content of the samples was determined by burning extracted samples in a muffle furnace for 90 minutes at 450
ºC and expressed the measured hormone levels as mass hormone/mass organic content in extracted faeces.
Feacal androgen metabolites were analyzed with a biotin-streptavidin enzyme immunoassay (EIA) first described by
Palme and Möstl [1994]. Antibodies were raised against 5α-androstane-3α-ol-17-one-
HS. 5α-androstane-3,17-dione-thioether was used as label and epiandrosterone was used as standard. Sample concentrations are expressed as µg/g organic dry content.
For the measurement of faecal estrogen metabolites a biotin-streptavidin
EIA was used with antibodies against 17βoestradiol-17-HS and oestrone was used as standard [Palme-Möstl, 1994]. Sample concentrations are expressed as ng/g organic dry content.
Faecal progestagen metabolites were analyzed by EIA of 5α-pregnan-3βol-20-on as described by Heistermann et al.
[1997] who used the assay for determining luteal activity in the African elephant
( Loxodonta Africana
). The 5α-pregnan-3βol-20-on concentrations was quantified by an EIA following Prakash et al. [1987] using a polyclonal antibody against 5αpregnan-3β-ol-20-one-3-HS-BSA and 5αpregnan-3β-ol-20-one-3-HS-peroxidase label. Sample concentrations are expressed as µg/g organic dry content.
The EIA used in this study to analyze GCM metabolite levels was an 11oxoaetiocholanolone first described by
Möstl et al. [2002]. This EIA detects GCM with a 3α-hydroxy-11-oxo structure and has successfully been validated for monitoring adrenocortical activity in various primate species, the African elephant, and other mammals [Möstl et al.,
2002; Ganswindt et al., 2003; Heistermann et al., 2006]. Sample concentrations are expressed as ng/g organic dry content.
The enzyme immunoassays for faecal androgen, estrogen, progestagen and
GCM metabolites levels were performed on microtiter plates with a double antibody technique according to the methods described by Palme and Möstl [1997] and
Ganswindt et al. [2002].
For the measurement of GCM metabolite levels in the blood samples a commercially available Coat-A-Count®
Cortisol radioimmunoassay from Siemens
Medical Solutions Diagnostics was used.
This is a solid-phase RIA wherein
125
Ilabeled cortisol competes for 45 minutes with the cortisol in the patient sample for antibody sites [Siemens Healthcare
Diagnostics, 2006].
Data Analysis
An independent sample t-test was used to test the androgen metabolite level found in the faecal samples of the aardwolves. The males and the females were compared on significant differences
4 C.M. Muilwijk, Utrecht University
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples in the means of their scores on the concentration androgen metabolite levels measured from the faecal samples.
For estrogen metabolites an independent sample t-test was used as well to compare the faecal concentration for significant differences between the males and females.
An independent sample t-test was used to control the manipulation of the progestagen metabolite level found in the faecal samples. The male aardwolves and the female aardwolves were compared on their scores on concentration progestagen metabolites measured from the faecal samples.
For the measurement of the glucocorticoid metabolite levels the
ACTH-challenge was accomplished. A ttest was used for the male (BWM09008) and the female (BWF08006) in question to compare the GCM metabolites levels in the faeces before and after the ACTH injection.
To measure the differences in immunoreactive cortisol in the blood for the specific male and the female, a paired sample t-test was done to check for significant differences pre and post injection for each aardwolf who had an injection. We crosschecked the immunoreactive cortisol blood levels with the GCM faeces metabolite levels for the
ACTH injection group to determine if these were the same or not and in which degree an increase was seen.
RESULTS
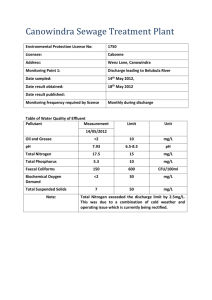
There were considerable differences between the studied aardwolves in terms of progestagen
(gestagen), androgen (testosterone) but not in estrogen metabolites (Table 1).
Table 1 Average hormone levels of the studied animals.
Animal ID Conc. Conc. Conc. Conc. GCM
Estrogen (ng/g (ng/g org) Progestagen
(µg/g org)
Androgen
(µg/g org) org)
BWF08006 34,364835 9,012874 54,110678 960,111460
BWF09009 43,264464 10,162596 64,302506 187,828617
BWM08002 6,502130 20,517373 73,443951 193,363149
BWM09008 11,526497 19,959667 66,031999 455,461890
Progestagen (gestagen)
Females had significantly higher faecal progestagen metabolite levels compared to males (Fig. 4). The average progestagen metabolite level ( M= 9.01) for the male samples were significantly different from the average progestagen metabolite level for the female samples
( M =38,11) ( t (64) = 4.90, p=0.000).
Androgen (testosterone)
Males had significantly higher faecal androgen metabolites levels compared to females (Fig. 5). The average for androgen metabolites for the male samples ( M =20,24) differed significantly from the average for female samples
( M =9,50), (t(64)=-3,41, p=0.001).
Estrogen
There were relatively marginal differences between the studied animals in faecal estrogen metabolites (Fig. 6). The average estrogen metabolite level ( M =
69,74 ) for the male samples were not significantly different from the average estrogen metabolite level for the female samples ( M =58,40), ( t (51) = -1,72, p=0.092).
5 C.M. Muilwijk, Utrecht University
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples
ACTH challenge
The mean GCM metabolite levels for the ACTH injection group ( M =763,86) differed significantly from the mean GCM metabolite levels from the non-ACTH injection group ( M =190,41), t(64)=-2,23, p=0.172. The glucocorticoid metabolites level increased significant in the faecal samples after the ACTH injection until 48 hours post injection for both the male
(BWM 09008) and the female
(BWF08006) (Fig 7). The ACTH injection yielded peak GCM metabolite levels in the faeces of 621% (female: 2129,92 ng/g org) and 542% (male: 1242,53 ng/g org) compared to pre-injection baseline. Mean pre-injection levels were 343,04 ng/g org and 229,28 ng/g org respectively for female and male. Peak GCM metabolite level for the female was measured in a fresh faecal sample 3 hours and 12 minutes after injection whereas peak glucocorticoid metabolite level for the male was measured
20 hours and 18 minutes after injection.
For both the female and the male, GCM metabolite levels returned to baseline within 44 hours (Fig. 7).
The injection yielded a significant increase in faecal GCM metabolite levels for both the male (t (13)= 2,68, p=.0.020) and for the female (t (21)= 2,71, p=0.048).
For the two studied animals the immunoreactive cortisol blood values and average GCM faeces values pre- and post
ACTH injection were established (Table
2). Faecal samples values increased 5,9x on average, for the blood values this was
5,5x on average.
By using a paired sample t-test we determined if the blood values before and after the ACTH injection were significantly increased. The mean beforehand (M=105,50) differed significantly from the mean afterwards
(M=571,66) t (1)= -46,8, p=0.14.
Table 2.
GCM blood and faecal values before and after ACTH injection.
BWF08006
BWM09008
BWF08006
BWM09008
Sample
Blood
Blood
Faeces
Faeces
DISCUSSION
The results showed that it was possible to determine androgen, progestagen and glucocorticoid metabolites in the faeces of aardwolves.
As expected androgen metabolites were significantly higher in the males than in the females and progestagen metabolites were significant higher in the female than in the males. We also expected that estrogen metabolites would be higher in the females
Pre-injection
155,3 nmol/l
55,7 nmol/l
343,04 ng/g org
Post-injection
631,4 nmol/l
511,9 nmol/l
2129,92 ng/g org
229,28 ng/g org 1242,53 ng/g org than in the males, but no significant difference was measured. A more comprehensive follow-up study should be done to investigate this.
The elevated GCM metabolite levels as a response to the ACTH challenge suggests that the measured metabolites are relevant indicators of adrenal activity.
However, the intensity of the physiologically induced stress response that we measured is different between the two aardwolves. A likely explanation for the mean peak of the female is that the animal was stressed prior the injection.
6 C.M. Muilwijk, Utrecht University
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples
In spotted hyenas ( Crocuta crocuta ), feacal glucocorticoid metabolites peaked 26 ± 5 hours after ACTH injection
[Goymann et al. 1999]. This is similar to our results on aardwolves, where feacal
GCM metabolites peaked approximately
20 hours after ACTH injection in the male and between 3 - 24 hours in the female.
CONCLUSION
In this study we demonstrated progestagen, androgen, estrogen and glucocoricoid metabolites levels in the faeces of four studied animal and therefore feaces collection can be used for noninvasive endocrine measurement in aardwolves.
Progestagen metabolites were significantly higher in the studied females and androgen metabolites were significantly higher in the studied males.
The elevated GCM metabolite levels in the feaces as a response to the
ACTH challenge presented that the measured metabolites are relevant indicators of adrenal activity. It can therefore be useful as a non-invasive tool for measuring a physiological stress response in this species.
ACKNOWLEDGEMENTS
I express my gratitude to F.
Dalerum and A. Ganswindt from the
Mammalian Research Institute, University
Pretoria for their guidance, encouragement and helpful advice during this project. I would also like to thank M. Engelkes from
University Utrecht for her support during the data collection. I thank M. Paris from
University Utrecht for giving me this opportunity. Finally I would like to thank
De Beers Consolidated Mines for permission to work on their property
Benfontein Game Farm.
REFERENCES
Cekan Z. 1975. Assessment of reliability of steroid radioimmunoassays. J Steroid
Biochem 6: 271-175.
Cook CJ, Mellor DJ, Harris PJ, Ingram JR,
Matthews LR. 2000. Hands-on and handsoff measurements of stress. IN: Moberg
GP, Mench JA. The Biology of Animal
Stress. Basic Principles and Implications for Animal Welfare. CAB International.
126-146.
Creel S, Creel NM, Monfort SL. 1996.
Social stress and dominance. Nature 379:
212.
Dloniak SM, French JA, Place NJ, Weldele
ML, Glickman SE, Holekamp KE.2004.
Non-invasive monitoring of fecal androgens in spotted hyenas ( Crocuta crocuta ). Gen Comp Endocrinol 135: 51-
61.
Fiess M, Heistermann M, and Hodges JK.
1999. Patterns of urinary and fecal steroid excretion during the ovarian cycle and pregnancy in the African elephant
( Loxodonta Africana ) . Gen Comp
Endocrinol 115: 76-89.
Ganswindt A, Heistermann M, Borragan S,
Hodges JK. 2002. Assessment of testicular endocrine function in captive African elephants by measurement of urinary and fecal androgens. Zoo Biol 21: 27-36.
Ganswindt A, Münscher S, Henley M,
Palme R, Thompson P, Bertschinger H.
2010. Concentrations of fecal glucocorticoid metabolites in physically injured free-ranging African elephants
( Loxodonta Africana ). Wildl. Biol. 16:
323-332.
Ganswindt A, Palme R, Heistermann M,
Borragan S, Hodges JK. 2003. Noninvasive assessment of adrenocortical
7 C.M. Muilwijk, Utrecht University
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples function in the male African elephant
( Loxodonta Africana ) and its relation to musth. Gen Comp Endocrinol 134:156-
166.
Goymann W, Mostl E, Van ’t Hof T, East
M, Hofer H. 1999. Noninvasive Fecal
Monitoring of Glucocorticoids in Spotted
Hyenas, Crocuta crocuta. Gen Comp
Endocrinol 114: 340-348.
Heistermann M, Palme R, Ganswindt A.
2006. Comparison of enzymeimmunoassays for assessment of andrenocortical activity in primates based on fecal analysis. IN: American J Primatol
68(3): 257-273.
Heistermann M, Trohorsch B, Hodges JK.
1997. Assessment of ovarian function in the African elephant ( Loxodonta africana ) by measurement of 5α-reduced progesterone metabolites in serum and urine. Zoo Biol 16: 273-284.
IUCN. 2008. IUCN red list of threatened species. http://www.iucn.org.
Koehler CE, Richardson PRK. 1990.
Mammalian Species, Proteles cristatus.
American Society of Mammalogists. 363:
1-6.
Monfort SL, Wildt DE, Holt W, Pickard A.
2002. Non-Invasive Endocrine Measures of Reproduction and Stress in Wild
Populations. IN: Reproduction and
Integrated Conservation Science.
Cambridge University Press, Cambridge,
UK. p. 147-165.
Möstl E, Maggs JL, Schrötter G,
Besenfelder U, Palme R. 2002.
Measurement of cortisol metabolites in faeces of ruminants. Vet Res Commun 26:
127-139.
Palme R, Möstl E. 1994. Proceedings 5th
Symposium on the Analysis of Steroids,
Budapest, Hungary. p 111–7.
Palme R, Möstl E. 1997. Measurement of cortisol metabolites in feaces of sheep as a parameter of cortisol concentrations in blood. Int J Mammal Biol 62: 192-197.
Prakash BS, Meyer HHD, Schallenberger
E, Van de Weil DFM. 1987. Development of a sensitive enzymimmunoassay (EIA) for progesterone determination in unextracted bovine plasma using the second antibody technique. J of Steroid
Biol 19: 383-392.
Schwarzenberger F, Möstl E, Palme R,
Bamberg E. 1996. Faecal steroid analysis for non-invasive monitoring of reproductive status in farm, wild and zoo animals .
Anim Repr Sci 42: 515-526.
Siemens Healthcare Diagnostics. Coat-A-
Count Cortisol. PITKCO-6. 2006-12-29.
Stenkewitz U, Kamler JF. 2008. Birds feeding in association with bat-eared foxes on Benfontein Game Farm, South Africa.
Ostrich 79: 235-237.
Taylor WA, Skinner JD. 2000. Associative feeding between Aardwolves (Proteles cristatus) and Aardvark (Orycteropus afer).
Mammal Rev 30(2): 141-143.
Williams JB, Anderson MD, Richardson
PRK. 1997. Seasonal differences in field metabolism, water requirements, and foraging behavior of free-living aardwolves. Ecology 78(8): 2588-2602.
8 C.M. Muilwijk, Utrecht University
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples
LEGENDS
Figure 4.
Gestagen concentrations in the feaces of the studied aardwolves.
Figure 5.
Androgen concentrations in the feaces of the studied aardwolves.
9 C.M. Muilwijk, Utrecht University
Non-invasive endocrine monitoring in the aardwolf (Proteles cristatus) using faecal samples
Figure 6.
Estrogens concentrations in the feaces of the studied aardwolves.
Figure 7.
Responses of immunoreactive GCM concentrations in faeces following IM injections of synthetic ACTH in two aardwolves.
7000
6000
5000
4000
3000
2000
1000
-56 -44 -32 -20 -8
0
4 16
Hours since AcTH injection
28 40
BWM09008
BWF08006
10 C.M. Muilwijk, Utrecht University