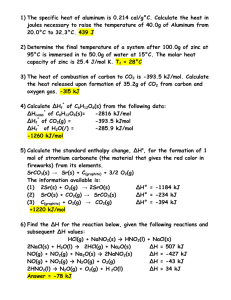
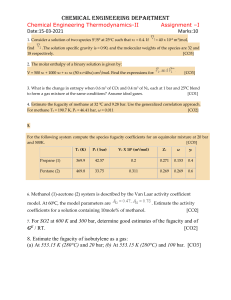
Sometimes, a reaction can take place in multiple steps instead of
advertisement

Sometimes, a reaction can take place in multiple steps instead of just one. Hess's Law says "the heat released or absorbed in a chemical process is the same whether the process takes place in one or in several steps." Example: A + B => AB AB + B => AB2 A + 2B => AB2 H1 H2 Htotal = H1 + H2 Steps to follow: 1. Balance the individual equations (if not balanced already) 2. Flip the equations around if necessary to cancel out terms on opposite sides 3. Changing the equation around requires a sign change of the H for that individual step. 4. One an equation needs to be multiplied by a number, multiple H for that individual step by that number. 5. Sum up the individual steps. Example: C(s, graphite) + O2 → CO2 ΔH = -393.5 kJ/mol CO2 → C(s, diamond) + O2 ΔH = + 395.41 kJ/mol C (s, graphite) => C (s, diamond) ΔH = ? Example: CH4 + 2O2(g) -> CO2(g) + 2H2O(l) H2O(l) -> H2O(g) H = -890 kJ/mol H = 44 kJ/mol at 298 K Calculate the enthalpy of the reaction CH4 + 2O2(g) -> CO2(g) + 2 H2O(g) H=? Example: Given: C(s) + O2(g) → CO2(g); ΔH = -393.5 kJ/mol S(s) + O2(g) → SO2(g); ΔH = -296.8 kJ/mol C(s) + 2 S(s) → CS2(l); ΔH = 87.9 kJ/mol What is the value for ΔH for the following reaction CS2(l) + 2 O2(g) → CO2(g) + 2 SO2(g) Example: N2H4(l) + CH4O(l) → CH2O(g) + N2(g) + 3H2 (g) ΔH = -37 kJ N2(g) + 3H2(g) → 2NH3(g) ΔH = -46 kJ CH4O(l) → CH2O(g) + H2(g) ΔH = -65 kJ Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: N2H4(l) + H2 => 2NH3(g)