Study Guide for the Fall Final The Scientific Method (Chapter 1
advertisement

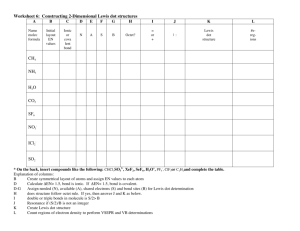
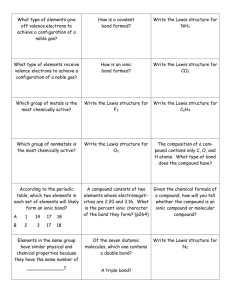
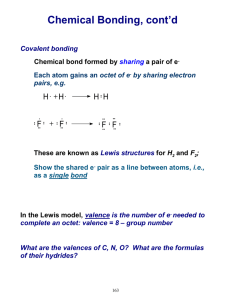
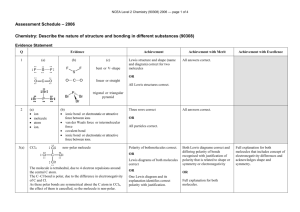
Study Guide for the Fall Final The Scientific Method (Chapter 1) 1. What it is the difference between a hypothesis, a theory and a law? 2. What is the process (steps) of the scientific method, and how did our experiment take us through these steps? Data Analysis and Scientific Notation (Chapter 2) 1. What are the base units for the metric (SI) system? What does each one of them measure? 2. What role do the prefixes play in the metric system? 3. Do problem #89 in Chapter 2 for scientific notation practice 4. What is the correct way to write in scientific notation? Write an example and explain what each number means. Atomic Structure (Chapter 4) 1. Which subatomic particle was found by JJ Thomson using a cathode ray tube? 2. What are the three subatomic particles and what are their charges? 3. What is the atomic number of an element? What is a mass number of an element? How are the two numbers different? 4. How do you calculate the number of neutrons in an element? The number of protons? If you are told the atom is neutral, can you calculate the number of electrons? Why or why not? If the atom is not neutral, can you calculate the number of electrons? Why or why not? 5. What caused the deflection in Rutherford’s gold foil experiment? What did Rutherford conclude about this particle? Electron Configuration (Chapter 5) 1. Be prepared to write out the electron configuration for any of the elements in the Periodic table from #1#118. You must write them out completely. 2. How many energy levels are there in the Periodic table? How do we find these levels using the Periodic Table? 3. What does each element in a Group have the same number of? 4. How can we use the electron configuration to find the valence elections? 5. Where on the periodic table are the s, p, d, and f-blocks? The Periodic Table (Chapter 6 & 7) 1. What are groups? What are periods? 2. What is the left-to-right trend for electronegativity? For atomic radius? For ionization energy? 3. What is the top-to-bottom trend for electronegativity? For atomic radius? For ionization energy? Ionic Bonding (Chapter 8) 1. What is an ionic bond? 2. What is a cation? How is it formed? 3. What is an anion? How is it formed? 4. What is the lattice structure of an ionic compound? 5. How do you name an ionic bond? Be prepared to name at least 10 different compounds, starting with 29-33 (pg 226) in your book. 6. How do you balance the charges on ionic compounds? 7. How would you balance the charges for problems 19-23 (pg 224 in your book) and problems 24-28 (pg 225) in your book? 8. Be prepared to draw the Lewis Dot Structure for Calcium Carbonate Covalent Bonding (Chapter 9) 1. What is a covalent bond? How is it formed? 2. What is a polar bond? Why is this bond polar? 3. How does electronegativity affect the type of bond? 4. What types of covalent bonds can be formed? 5. What is a nonpolar molecule? How is it formed? 6. What are the charges on each side of a polar molecule? 7. Why do polar solvents dissolve polar solutes and non-polar solvents dissolve non-polar solutes? 8. Why are the bonds in biological molecules covalent? (Hint: it is not because they are “strong” bonds. A couple of groups had this correct; most did not) 9. What is the shape of a covalently-bound molecule if you are using VSEPR? 10. What is a “lone pair”? How does this pair affect the shape of a covalently-bound molecule? Lewis Dot Structures 1. What is the octet rule? Why must this be fulfilled? How can you show this being filled on a Lewis Dot Structure? 2. When drawing Lewis Dot Structures, remember that you have to fill all of the octets of all of the elements involved in bonding. So, if you don’t have enough electrons, you might need to have more than one element. Example: I gave you Al Cl on the quiz. Al has 3 valence electrons, but Chlorine has 7 and only needs one. That means you would need 2 more Chlorines for a total of 3. The formula would be AlCl3 and the Lewis Dot Structure would be Al surrounded by 3 Cl. Chapter 10: Chemical Reactions 1. What is a chemical reaction? How can you tell that one has taken place? 2. What are the five types of Chemical Reactions? How are the atoms rearranged in each type? 3. What is the Law of the Conservation of Mass and why does it make us balance chemical equations? 4. Be prepared to balance any chemical equation. (Insert evil laughter here) Chapter 11: Ze Mole 1. What is Avogadro’s number? What does that number represent? 2. What is ze mole? What does it measure? 3. How do we use the periodic table to convert grams to moles and moles to grams? 4. How do we use Avogadro’s number to convert moles to particles to moles? 5. How do we use both Avogadro’s number to covert grams to particles and particles to grams? 6. Be prepared to do mole calculations 7. What is percent composition? What does it tell us? 8. Be prepared to calculate percent composition problems. 9. What is empirical formula? 10. What is molecular formula? 11. Be prepared to calculate both. Chapter 12: Stoichiometry 1. What is stoichiometry? 2. What is a limiting reactant? 3. What is an excess reactant? 4. What is percent yield and why do we have to calculate it? 5. Be prepared to do stoichiometry problems including limiting/excess reactant problems and percent yield problems. Good luck!