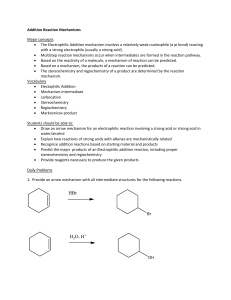
Energy Diagrams and kinetics
advertisement
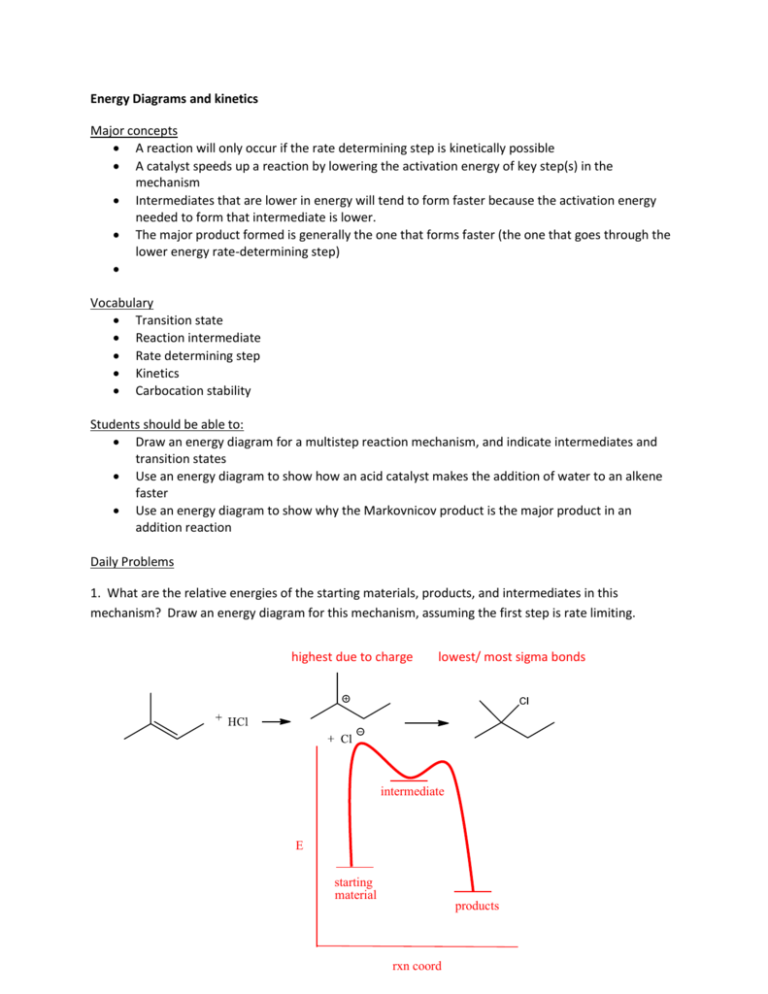
Energy Diagrams and kinetics Major concepts A reaction will only occur if the rate determining step is kinetically possible A catalyst speeds up a reaction by lowering the activation energy of key step(s) in the mechanism Intermediates that are lower in energy will tend to form faster because the activation energy needed to form that intermediate is lower. The major product formed is generally the one that forms faster (the one that goes through the lower energy rate-determining step) Vocabulary Transition state Reaction intermediate Rate determining step Kinetics Carbocation stability Students should be able to: Draw an energy diagram for a multistep reaction mechanism, and indicate intermediates and transition states Use an energy diagram to show how an acid catalyst makes the addition of water to an alkene faster Use an energy diagram to show why the Markovnicov product is the major product in an addition reaction Daily Problems 1. What are the relative energies of the starting materials, products, and intermediates in this mechanism? Draw an energy diagram for this mechanism, assuming the first step is rate limiting. highest due to charge lowest/ most sigma bonds intermediate E starting material products rxn coord 2. Draw an energy diagram for a 2-step mechanism with a high-energy intermediate that is endothermic. (Assume its first step is the rate determining step.) The intermediate is higher than the starting materials or products. The highest transition state is the first one, representing the rate determining step. The products are higher than the starting material, indicating the exothermic reaction. intermediate E products starting material rxn coord 3. Draw an energy diagram for a spontaneous three-step reaction mechanism in which the intermediates are high energy intermediates and the second transition state is the highest energy TS. Which step is rate determining? Spontaneous: starting materials are higher in energy than the products. The rate determining step is from the first intermediate to the second intermediate because it is the highest transition state. E rxn coord 4. Draw energy diagrams for mechanisms A and B for the addition of water to propene. (Be sure to consider the relative stabilities of all the compounds and draw them with correct relative energies.) Which reaction works? What effect does the catalyst have on the reaction? Refer to your energy diagrams to answer these questions. Mechanism B works while mechanism A does not because the rate determining step in A is too high so the reaction is very slow! The addition of a catalyst in B lowers the RDS transition state/Ea and speeds up the reaction Mechanism A: Mechanism B: E E rxn coord rxn coord 5. A. Draw an energy diagram for just the first step (the rate determining step) of the addition of HCl to propene to form the primary carbocation. B. Draw a similar diagram for formation of the secondary carbocation. C. Put parts A and B together on the same diagram, taking into consideration that the starting material is identical in both cases. D. How does this energy diagram explain why the Markovnicov product is formed rather than the primary alkyl chloride? This diagram shows you that the formation of the primary carbcation (red line) takes more energy because it is less stable, while the formation of the secondary carbocation (blue line) takes less energy because it is more stable. E rxn coord Cumulative problems 6. Draw mechanisms for the formation of Product A and Product B. Explain why Product A is formed, but not product B using an energy diagram. Product A is formed over product B because the activation energy (height of the RDS transition state) is lower to form a resonance stabilized carbocation (red) over a nonstabilized carbocation (blue). E rxn coord 7. When HBr is added to 1,1,1-trifluorobut-2-ene, two possible carbocation intermediates could form. Draw both possible intermediates. Which one is more stable? Draw the major product of the reaction. 8. When treating a diene with HCl, only one potential intermediate cation is formed, and not the other. Explain. HCl This carbocation is resonance stabilized not formed Once this intermediate is formed, it can lead to two products. Explain, and give a mechanism. Extension problem 9. Based on the bonding in this reaction, in which direction would you predict the equilibrium of this reaction to lie? This is a non-ionic reaction, so you must evaluate the bonds made to the bonds broken. Broken: C-OH σ and C-H σ Made: C=C π and H-OH σ Sigma bonds are more stable than pi bonds; the reaction would lie to the side with more sigma bonds. The equilibrium lies to the left. If, during the course of this reaction, water is constantly removed so that it can’t react to go back to starting material, the equilibrium of the reaction would be force to the right. This is based on La Chatlier’s principle (see your C117 notes or textbook). If there is no water to add across the pi bond, there is no H-X reactant and no reverse reaction.