NOTES - Unit 7 Covent bonding
advertisement
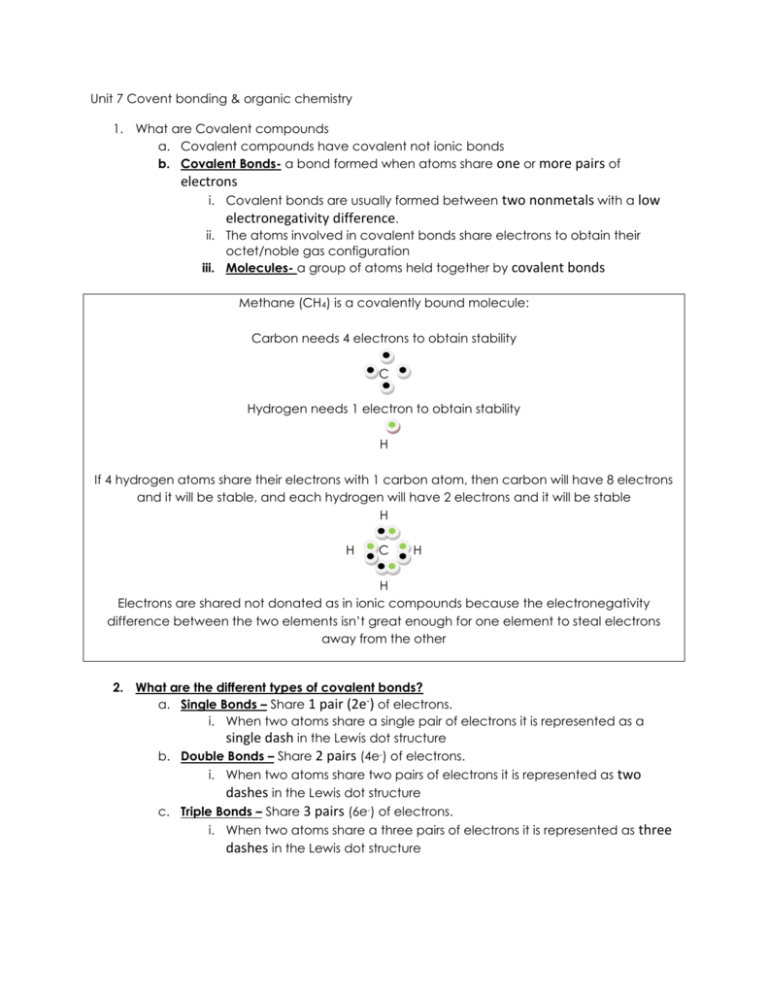
Unit 7 Covent bonding & organic chemistry 1. What are Covalent compounds a. Covalent compounds have covalent not ionic bonds b. Covalent Bonds- a bond formed when atoms share one or more pairs of electrons i. Covalent bonds are usually formed between two nonmetals with a low electronegativity difference. ii. The atoms involved in covalent bonds share electrons to obtain their octet/noble gas configuration iii. Molecules- a group of atoms held together by covalent bonds Methane (CH4) is a covalently bound molecule: Carbon needs 4 electrons to obtain stability C Hydrogen needs 1 electron to obtain stability H If 4 hydrogen atoms share their electrons with 1 carbon atom, then carbon will have 8 electrons and it will be stable, and each hydrogen will have 2 electrons and it will be stable H H C H H Electrons are shared not donated as in ionic compounds because the electronegativity difference between the two elements isn’t great enough for one element to steal electrons away from the other 2. What are the different types of covalent bonds? a. Single Bonds – Share 1 pair (2e-) of electrons. i. When two atoms share a single pair of electrons it is represented as a single dash in the Lewis dot structure b. Double Bonds – Share 2 pairs (4e-) of electrons. i. When two atoms share two pairs of electrons it is represented as two dashes in the Lewis dot structure c. Triple Bonds – Share 3 pairs (6e-) of electrons. i. When two atoms share a three pairs of electrons it is represented as three dashes in the Lewis dot structure 3. What are the properties of covalent bonds? a. Molecular substances that consist of covalently bound atoms have a low melting point, they are usually brittle, and they have a strong odor, b. Because there are charged ions molecular substances are poor conductors of electricity. c. because atoms in carbons family can bond so many times, covalent compounds can form isomers d. Isomers- Molecules that are made out of the same atoms but are different 3 dimensional shapes i. Even through the formulas of isomer molecules are exactly the same, the unique three dimensional shape of an isomer gives it unique sets of chemical and physical properties like boiling point Isomer (C8H18) CH3-CH2-CH2-CH2-CH2-CH2-CH2-CH3 Number of branches 0 Boiling point 125oC 1 117oC 3 99oC a. As you can see, even though all the isomers have the same formula, the more branches they have the lower the boiling point of that isomer 2. How covalent compounds are represented a. There are three ways to represent organic molecules: i. Molecular/chemical formula – describe the number and type of atoms in the molecule 1. In a molecular formula the atoms are listed in C, H, O, N order C2H6O CH4ON2 CHN ii. Structural formula – describes the way the atoms In the molecule are linked together 1. Every covalent bond in a structural formula is indicated with a dash 2. in compounds that have a lot of carbon, sometimes a skeletal formula is used in p[lace of a structural formula 3. skeletal formulas don’t draw hydrogen and replace carbons with kinks iii. Valance dot diagram – shows the atoms in the molecule share electrons. 4. How do you go from a formula to a name with a covalent compound? a. First check to see if it is a covalent compound be seeing if it is a metal bound to a nonmetal or two non metals bound together i. If it is two non metals then it is probably covalent ii. If it is metal bound to a non metal than it is ionic iii. If there is a polyatomic anion involved then it is ionic b. Covalent compounds are all named with prefixes that tell how many of each type of atom are present in the molecule: Mono- = 1 atom Hexa- = 6 atoms Di- = 2 atoms Hepta- = 7 atoms Tri- = 3 atoms Octa- = 8 atoms Tetra- = 4 atoms Nona- =9 atoms Penta- = 5 atoms Deka- = 10 atoms c. The only time you don’t use a prefix is if there is 1 of the first atom: Photsphorous triodide = PI3 VS. Diphosphorous trioxide = P2O3 Write the formula of the following covalent compounds carbon monoxide phosphorus tribromide carbon tetrachloride diphosphorus trioxide nitrogen trichloride 5. How do you go from a name to a formula with a covalent compound? a. First determine if it an ionic or covalent compound: i. If it’s a metal bound to a non metal its ionic ii. If its two non-metals bound together its covalent b. Simply write the symbol for each element in the order it appears with a subscript after that corresponds to the prefix i. Remember the only time you don’t use a prefix is if there is one of the first element Name the following covalent compounds: SiO2 SO3 SiF4 N2O PCl3 1. Organic Chemistry a. Organic Chemistry- “carbon” chemistry, the study of those substances made up of carbon. i. Since most of the molecules organisms use to live are made out of carbon, the study of molecules containing carbon is in a way studying the chemistry of life ii. Hydrocarbons- molecules made out of carbon and hydrogen 1. All hydrocarbon molecules have a carbon chain as their backbone a. Carbon Chain- in hydrocarbon molecules carbon atoms may link-up to form large molecules with this as their “backbone.” 2. How to name organic compounds a. To name organic compounds you first have to count and number the carbons in the backbone b. Then use a prefix to indicate the number of carbons in the backbone i. Meth = 1 carbon ii. Eth = 2 carbon iii. iv. v. vi. Prop= 3 carbon But- = 4 carbon Pent- =5 carbon Hex- = 6 carbon vii. viii. ix. x. Hept- = 7 carbon Oct- = 8 carbon Non- = 9 carbon Dec- = 10 carbon c. Use a suffix to indicate if the hydrocarbon is situated or had double or triple bonds i. –ane = all carbons are single bond to each other ii. –ene = one or more carbons are double bound to each other iii. –yne = one or more carbons are triple bound to each other d. Last right the number of the carbon that the double or triple bond takes place, and or the functional group Ethyne Ethane Butane Ethene 1-butene 2-butene 2. What are alkanes? a. Alkanes – a hydrocarbon molecule that consists of only single bonded carbon atoms. iv. Each carbon is covalently bonded to 4 other atoms in an alkane. v. Alkanes are considered to be a saturated hydrocarbon as each carbon atom is bonded to a maximum number of other atoms (4). 1. The simplest alkane is methane CH4, however you can begin to get a longer and longer carbon chain and surround it by carbon atoms 2. To determine the chemical formula of any Alkane Series Pattern obeys the equation: CnH2n+2 Ex) C2H6 C3H8 C35H 3. What are Alkenes a. In some hydrocarbon molecules, carbon atoms bond to 3 other atoms, not four. i. Alkenes – a hydrocarbon molecule that consists of a single or multiple carbon with double bonds 1. The general formula for Alkenes is Cn H2n a. Ex) C3H6 C18H36 C50H100 Alkane Vs Alkene Vs. 2. With alkenes there are even more opportunities to have isomers 4. What are Alkynes a. In some hydrocarbon molecules, carbon atoms are bond to 2 other atoms, not four. i. Alkynes – a hydrocarbon molecule that consists of a single or multiple carbon with a triple bond 1. In a triple covalent bond there are 6 electrons shared between the two bonding atoms. a. General formula for Alkynes is CnH2n-2 b. Ex) C3H4 C18H34 C50H98 5. What happens when carbon forms a ring? a. Organic molecules don’t only have to be chains they can also be rings i. Cycloalkanes- are single-bonded hydrocarbons in rings Vs. b. When rings are unsaturated (have a double bond) they are called aromatic compounds i. Aromatic compounds – unsaturated carbon rings 6. Functional groups a. Organic molecules are recognized by their functional group, i. Functional group - which is an atom or group of atoms that imparts characteristic properties to the organic molecules. 1. There are 4 different functional groups that involve an oxygen atom a. Alcohols b. Ethers c. Esters d. Carboxylic acids 7. Alcohols a. Alcohol - functional group, that consists of oxygen bound to hydrogen on one side. H2 i. Alcohols are represented as -OH. C H3C OH Methanol H3C CH2 OH Ethanol (ethyl alcohol) CH3CH2CH2OH 1-propanol H2C CH2 H2C CH CH2 OH cyclohexanol ii. Chemical and physical properties of alcohols: 1. Alcohols have an odor that is often described as “biting” and as “hanging” in the nasal passages. 2. Alcohols are also polar 8. Ethers a. Ether- functional group that consists of a oxygen bound to a carbon on both sides i. Ethers are represented by –O- . ii. Chemical and physical properties of ethers iii. Chemical and physical properties of ethers 1. Ethers tend to be non-polar 2. Ethers has have a low melting point 9. Carboxylic Acid a. Carboxylic Acid- a functional group, that consists of a carbon atom that has a double covalent bond to one oxygen atom and a single covalent bond to an alcohol i. Carboxylic acids are represented by -COOH ii. Carboxylic acids chemical and physical properties 1. Carboxylic acids are polar 2. High boiling point 3. Weak acids 4. Strong and volatile Oder 10. Ester a. Ester- a functional group, that consists of a carbon atom that has a double covalent bond to one oxygen atom as well as an ether i. Esters are represented by -COO- group attached.







