APl supplementary info final
advertisement
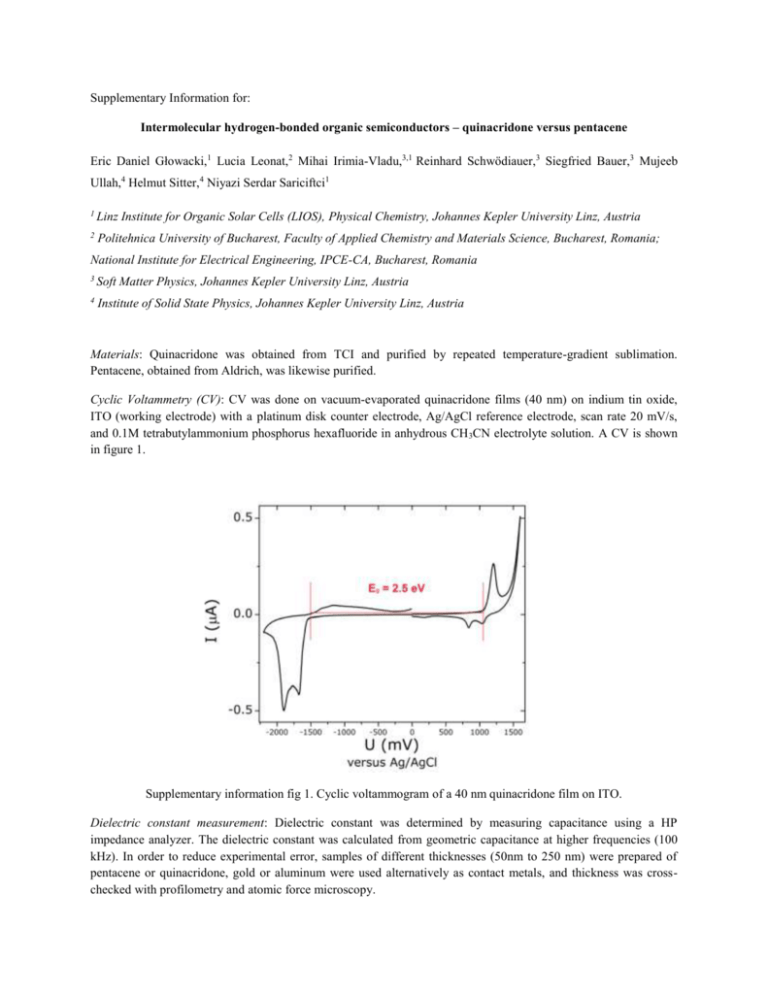
Supplementary Information for: Intermolecular hydrogen-bonded organic semiconductors – quinacridone versus pentacene Eric Daniel Głowacki,1 Lucia Leonat,2 Mihai Irimia-Vladu,3,1 Reinhard Schwödiauer,3 Siegfried Bauer,3 Mujeeb Ullah,4 Helmut Sitter,4 Niyazi Serdar Sariciftci1 1 Linz Institute for Organic Solar Cells (LIOS), Physical Chemistry, Johannes Kepler University Linz, Austria 2 Politehnica University of Bucharest, Faculty of Applied Chemistry and Materials Science, Bucharest, Romania; National Institute for Electrical Engineering, IPCE-CA, Bucharest, Romania 3 Soft Matter Physics, Johannes Kepler University Linz, Austria 4 Institute of Solid State Physics, Johannes Kepler University Linz, Austria Materials: Quinacridone was obtained from TCI and purified by repeated temperature-gradient sublimation. Pentacene, obtained from Aldrich, was likewise purified. Cyclic Voltammetry (CV): CV was done on vacuum-evaporated quinacridone films (40 nm) on indium tin oxide, ITO (working electrode) with a platinum disk counter electrode, Ag/AgCl reference electrode, scan rate 20 mV/s, and 0.1M tetrabutylammonium phosphorus hexafluoride in anhydrous CH 3CN electrolyte solution. A CV is shown in figure 1. Supplementary information fig 1. Cyclic voltammogram of a 40 nm quinacridone film on ITO. Dielectric constant measurement: Dielectric constant was determined by measuring capacitance using a HP impedance analyzer. The dielectric constant was calculated from geometric capacitance at higher frequencies (100 kHz). In order to reduce experimental error, samples of different thicknesses (50nm to 250 nm) were prepared of pentacene or quinacridone, gold or aluminum were used alternatively as contact metals, and thickness was crosschecked with profilometry and atomic force microscopy. Supplementary information fig 2. Dielectric constant (r) as a function of probing frequency on the left axis, loss angle on the right axis. Organic field effect transistor (OFET) fabrication details: Transistors were fabricated on glass substrates by first creating an AlOx dielectric layer electrochemically1 (40 nm) on a vacuum-evaporated aluminum gate electrode (100 nm). Next, a 40 nm passivation layer of the oligoethylene tetratetracontane (C 44H90, TTC) was evaporated onto the AlOx. Easily extracted from petroleum distillation, TTC is biodegradable and occurs abundantly in nature, being identified in large amounts in medicinal plants 2,3,4,5 TTC-passivated AlOx slides were annealed at 60 oC for ~12h under nitrogen6, giving a specific capacitance, C0d, of 43.2 nF·cm-2. Next, 80 nm of Quinacridone (or pentacene) was evaporated at a rate of 0.1 Å/s. Gold source and drain electrodes were evaporated through a shadow mask giving W/L of 2 mm / 75 µm. Supplementary information fig 3. Transfer characteristics of a quinacridone OFET (a) and a pentacene OFET (b). Pentacene on the TTC/AlOx dielectric shows ambipolarity. Ambipolarity in pentacene has been reported in the literature (see ref. 7). Leakage currents (Igs) were in the sub-nanoamp regime for all devices. Thin film X-Ray diffraction (XRD): fig.4. (a) shows the x-ray diffraction of quinacridone films. The broad amorphous peak is originating from the glass substrate. (b) A packing diagram of quinacridone in the γ phase with the [002] plane shown, corresponding to the diffraction peak at 2θ=6.5°. The unit cell is viewed down the b-axis, with a slight tilt along this axis to show how the molecules stack on top of each other. Single crystal diffraction data is from ref. 8. Supplementary information fig 4. XRD results. Diode devices: For diode devices, a structure of ITO-coated glass (30Ω/sq.) covered with 40 nm of Baytron PEDOT:PSS was used as the substrate/bottom contact. A 100 nm layer of the organic semiconductor was used. Both organic materials were evaporated at a rate of 0.1 Å s-1 and at a pressure of 1×10-6 torr. Metal top contacts were evaporated through a shadow mask with a rate of 1 Å s-1 at a pressure of 1×10-6 torr. Measurements were conducted in an N2-filled glove box. Storage of samples for at least 6 months in ambient air resulted in little degradation of performance. This is attributed to the known stability of quinacridone pigments. Thermally-activated photocurrent: Contacts with 1 mm width and 0.5 mm spacing were evaporated onto a ~80 nm film of quinacridone on glass and the photocurrent under green laser illumination was measured. For each temperature, a dark current was measured followed by a light current, with the difference constituting the photocurrent. A field of 200 V/cm was used, different contact metals (i.e. Ag, Au) showed no influence on the experiment. Measurements were conducted under vacuum in an Oxford Instruments cryostat. An Arrhenius plot is shown in figure 5. On the basis of this, we estimate an effective exciton binding energy of 12 ± 5 meV. Supplementary information fig 5. Arrhenius plot of the thermally-activated photocurrent in quinacridone films. 1 L.A. Majewski, M. Grell, S.D. Ogier, J. Veres, Org. Electron. 4, 27 (2003). J.R. Haines, M. Alexander, Appl. Microbiol. 28, 1084-1085 (1975). 3 G.K. Oloyede, I. A. Oladosu, A. F. Shodia, African J. Pure Appl.Chem. 4, 35 (2010). 4 N.S. Muslim, K.W. Ng, A. Itam, Z.D. Nassar, Z. Ismail, A.M.S. Ablul Majid, Int. J. Pharmacol. 6, 591 (2010). 5 P.S: Hariprasad, N. Ramakrishnan, Int. J. Drug Develop. Res. 3, 272 (2011). 6 A. Kraus, S. Richler, A Opitz, W. Brütting, S. Haas, H. Tatsuo, A. Hinderhofer, F. Schreiber, J. Appl. Phys. 107, 094503 (2010). 7 T.B. Singh, F. Meghdadi, S. Günes, N. Marjanovic, G. Horowitz, P. Lang, S. Bauer, and N.S. Sariciftci, Adv. Mater. 17, 2315 (2005). 8 N. Nishimura, T. Senju, and J. Mizuguchi, Acta Cryst. E. 62, 4683 (2006). 2