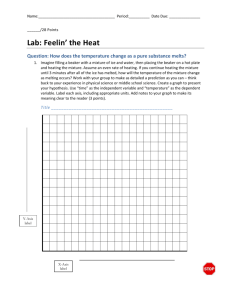
Problem SM.5 Questions
advertisement
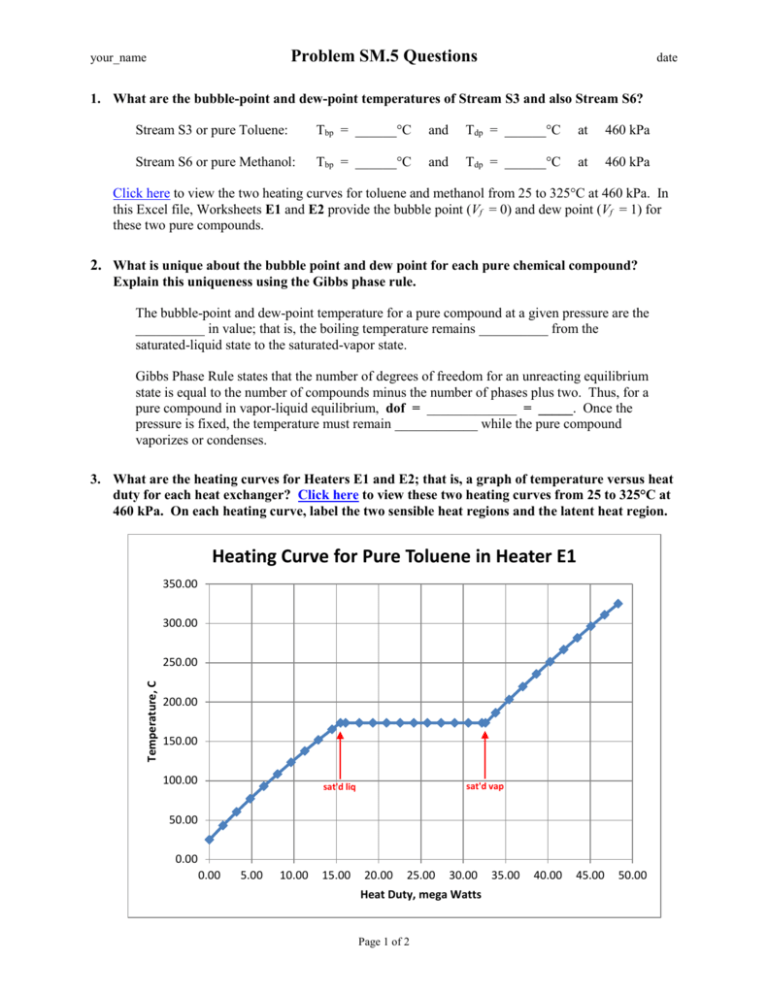
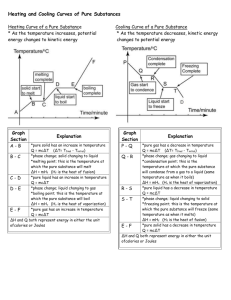
Problem SM.5 Questions your_name date 1. What are the bubble-point and dew-point temperatures of Stream S3 and also Stream S6? Stream S3 or pure Toluene: Tbp = ______°C and Tdp = ______°C at 460 kPa Stream S6 or pure Methanol: Tbp = ______°C and Tdp = ______°C at 460 kPa Click here to view the two heating curves for toluene and methanol from 25 to 325°C at 460 kPa. In this Excel file, Worksheets E1 and E2 provide the bubble point (Vf = 0) and dew point (Vf = 1) for these two pure compounds. 2. What is unique about the bubble point and dew point for each pure chemical compound? Explain this uniqueness using the Gibbs phase rule. The bubble-point and dew-point temperature for a pure compound at a given pressure are the __________ in value; that is, the boiling temperature remains __________ from the saturated-liquid state to the saturated-vapor state. Gibbs Phase Rule states that the number of degrees of freedom for an unreacting equilibrium state is equal to the number of compounds minus the number of phases plus two. Thus, for a pure compound in vapor-liquid equilibrium, dof = _____________ = _____. Once the pressure is fixed, the temperature must remain ____________ while the pure compound vaporizes or condenses. 3. What are the heating curves for Heaters E1 and E2; that is, a graph of temperature versus heat duty for each heat exchanger? Click here to view these two heating curves from 25 to 325°C at 460 kPa. On each heating curve, label the two sensible heat regions and the latent heat region. Heating Curve for Pure Toluene in Heater E1 350.00 300.00 Temperature, C 250.00 200.00 150.00 100.00 sat'd vap sat'd liq 50.00 0.00 0.00 5.00 10.00 15.00 20.00 25.00 30.00 Heat Duty, mega Watts Page 1 of 2 35.00 40.00 45.00 50.00 Problem SM.5 Questions your_name date Heating Curve for Pure Methanol in Heater E2 350.00 300.00 Temperature, C 250.00 200.00 150.00 100.00 50.00 sat'd vap sat'd liq 0.00 0.00 5.00 10.00 15.00 20.00 25.00 30.00 35.00 40.00 Heat Duty, mega Watts 4. What is the slope of the heating curve in the transition region from the saturated-liquid state to the saturated-vapor state? The slope of the latent heat portion of the heating curve is __________; that is, it is _____________ from the saturated-liquid state to the saturated-vapor state. Based on the Gibbs phase rule, the temperature must remain ____________ for a given pressure while a pure chemical compound is vaporizing or condensing. Page 2 of 2