0581.5271 – “Electrochemistry for Engineers” HOMEWORK #5

0581.5271 – “Electrochemistry for Engineers”
HOMEWORK #5
Question 1) Consider a PEM fuel cell with a Pd anode and a Ru x
Se y
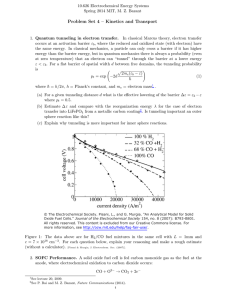
cathode and a Nafion membrane that is 10cm 2 operating at 25°C with pure oxygen and hydrogen inputs at 1 ATM each. a) Derive analytical expressions that relate the fuel cell’s operating voltage to the current density obtainable from the cell by considering the ohmic and activation losses (consider the cathode and the anode separately). b) Using a computer, plot a current density vs. voltage graph to show the effect of ohmic, cathodic overpotential, and anodic overpotential as a function of current density between 0 and 2mA/cm 2 . State all of your assumptions.
Start by showing the open circuit voltage line, then show a new line after each consecutive loss is accounted for. In other words, there should be 4 lines.
(1=OCP, 2=OCP-η ohm
, 3=OCP- η ohm
- η act, cath
, 4= OCP - η ohm
- η act, cath
- η act, anode
) c) Will increasing the flow rate of oxygen and hydrogen flow rates to “infinity” create a fuel cell that can output any current desired? Give a detailed explanation as to why or why not.
Question 2) A direct methanol fuel cell (DMFC) utilizes methanol (CH3OH) instead of hydrogen as its fuel.
The overall cell reaction is:
CH
3
OH
(liq)
+ 3/2 O
2
↔ CO
2
+ 2H
2
O
(liq) a) Using the data below, calculate the open circuit potential of the cell at 45°C
Chemical Species
CH
3
OH
(liq)
O
2
CO
2
H
2
O
(liq)
ΔH (kJ/mol) @ 45
°
C
-238.4
0
-393.51
-258.83
S (kJ/mol) @ 45
°
C
127.19
205.14
213.8
69.95
b) Assume that the DMFC described above is operating at half of its open circuit potential at 45°C, how much energy (in kJ/mol) is being lost to heat?
Question 3) Explain in 4-5 sentences why a laminar flow fuel cell needs to operate with a narrow channel and a fast velocity of fuel and oxidant? What variable is used to find the upper limit of the velocity? Use the paper by the
Kenis group to help you answer. You do not need to read section 3.2 or after.