Example
advertisement

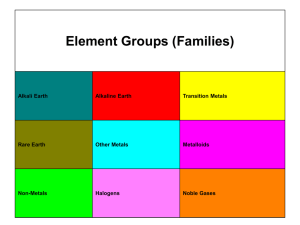
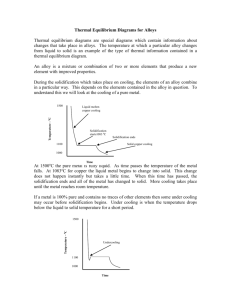
Type -3- Two metals, completely soluble in all proportion in the liquid state, are only partially soluble in the solid state Since most metals show some solubility each other in the solid state, this type is the most common and therefore, the most important alloy system Fig -10- Phases diagram illustrating partial solid solubility The melting points of the two pure metals are indicated at points TA and TB, respectively. The liquidus line is TA E TB, and the solidus line is TA F E G TB. Above the liquidus line, there is only a single phase liquid solution. These metals (metal A and metal B) are partly soluble in the solid state, a solid solution must be formed. Alloys in this system never solidify crystal of pure A or pure B but always a solid solution or mixture of solid solutions. At TE, the solid solution dissolves a maximum of 20 % B as shown by point F, and the B solid solution a maximum of 10 % A as shown by point G. With decreasing temperature, the maximum amount of solute that can be dissolved decreasing, as indicated by lines FH and GJ. These lines are called solvus lines and indicate the maximum solubility (saturated solution) of B in A ( - solution) or A in B (B solution) as a function of temperature point E, where the liquidus lines meet at a minimum is known as the (eutectic point). Example Draw cooling curve of alloy (1) (Fig. 10) Fig -11- The cooling curve and microstructure at various temperature during solidification of 95 % A – 5 % B alloy As example: Example Determine the relative amounts of mixture (Fig. 10) and B in the eutectic Solution The relative amounts of and B in the eutectic mixture may be determined by applying (lever rule) EG 100% FG EF %B 100% FG % 20 100 28.6% 70 50 100% 71.4% 70 Example Determine the relative amounts of point K. (Fig. 10) and β at room temperature, Solution KJ 95 70 100% 100 29.4% HJ 95 10 HK 70 10 B% 100% 100 70.6% HJ 95 10 % Example Write the eutectic reaction (from Fig. 10) Solution This reaction is the same as the one which occurred in the type (2) of thermal equilibrium diagram, except for the substitution of solid solution for pure metals. Because of the change in solubility of metal B in metal A, line FH, and of metal A in metal B, line GJ, there will be a slight change in the relative amounts of and B as the alloy is cooled to room temperature. The relative amounts of and B at room temperature are. KJ 25 100% 100 29.4% HJ 85 HK 60 B% 100 100 70.6% HJ 85 % Example Draw cooling curve of alloy 3 (Fig. 10) Fig -12- The cooling curve and microstructure at various temperature during solidification of alloy 3 (60 % A + 40 % B)