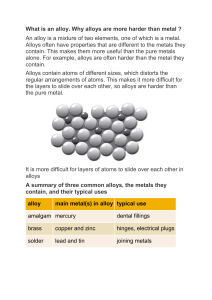
Metallic Bonds
advertisement
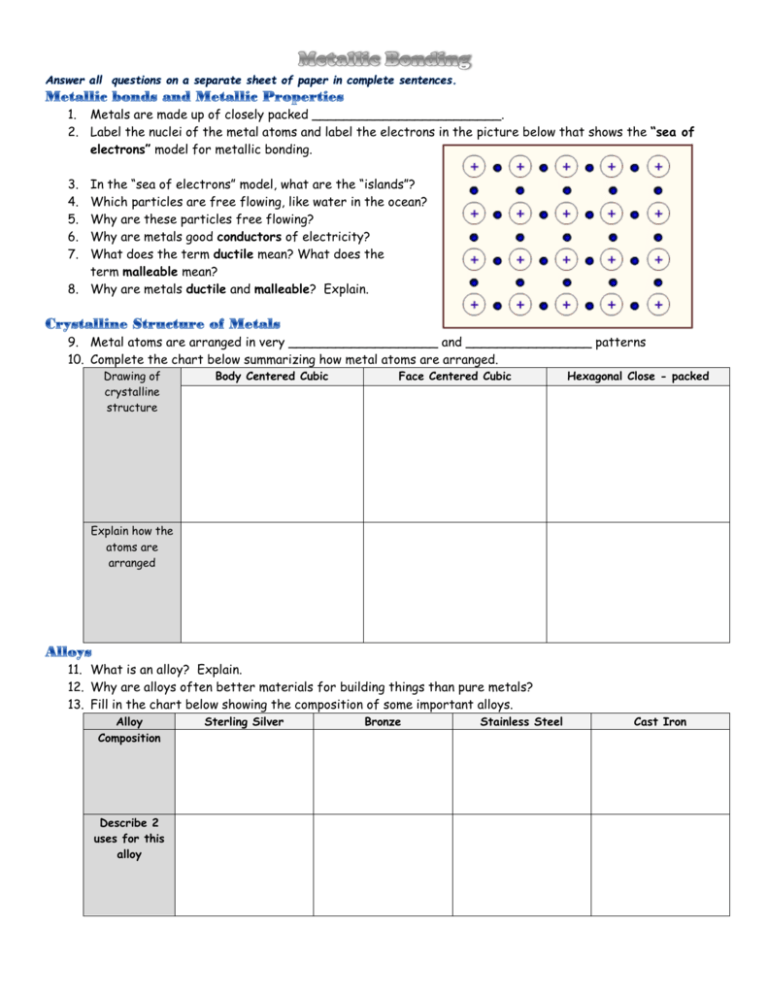
1. Metals are made up of closely packed ________________________. 2. Label the nuclei of the metal atoms and label the electrons in the picture below that shows the “sea of electrons” model for metallic bonding. 3. 4. 5. 6. 7. In the “sea of electrons” model, what are the “islands”? Which particles are free flowing, like water in the ocean? Why are these particles free flowing? Why are metals good conductors of electricity? What does the term ductile mean? What does the term malleable mean? 8. Why are metals ductile and malleable? Explain. 9. Metal atoms are arranged in very ___________________ and ________________ patterns 10. Complete the chart below summarizing how metal atoms are arranged. Drawing of crystalline structure Body Centered Cubic Face Centered Cubic Hexagonal Close - packed Explain how the atoms are arranged 11. What is an alloy? Explain. 12. Why are alloys often better materials for building things than pure metals? 13. Fill in the chart below showing the composition of some important alloys. Alloy Composition Describe 2 uses for this alloy Sterling Silver Bronze Stainless Steel Cast Iron