a.primary phagocyte deficiencies
advertisement

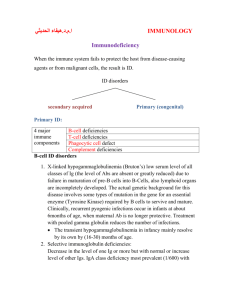
1 IMMUNE DEFICENCY IMMUNE DEFICIENCY CLINICAL FEATURES : 1. Recurrent infections: Frequent, severe infections caused by unusual organisms & at unusual sites 2. Autoimmunity 3. Susceptibility to malignancy CAUSES: I. PRIMARY IMMUNE DEFICIENCY: A.PRIMARY PHAGOCYTE DEFICIENCIES Majority present in childhood. Types: 1) Leucocyte adhesion deficiencies: Disorders of phagocyte migration Sites of infection lack pus (neutrophil infiltration) Peripheral blood neutrophil counts may be very high during acute infection 2) Chronic granulomatous disease Results from mutations in the genes encoding the NADPH oxidase enzyme, causing a failure of oxidative killing leads to susceptibility to catalase-positive organisms such as Staphylococcus aureus, Burkholderia cenocepacia and aspergillus Intracellular killing of mycobacteria in macrophages is also impaired Infections most commonly involve the lungs, lymph nodes, soft tissues, bone, skin and urinary tract and are characterised histologically by granuloma formation May be demonstrated using the nitroblue tetrazolium reduction test (NBT), which measures the ability to reduce a colourless intracellular dye to an insoluble blue compound after neutrophil activation IMMUNE DEFICENCY 2 3) Defects in cytokines and cytokine receptors Defects of cytokines such as IFN-γ, IL-12 or their receptors result in failure of intracellular killing Individuals are particularly susceptible to mycobacterial infections Management : 1. Drugs: Intravenous antibiotics, Longterm prophylaxis with antifungal agents, and trimethoprim-sulfamethoxazole. 2. Surgical drainage of abscesses 3. Specific treatment depends upon the nature of the defect, and stem cell transplantation may be considered. B. COMPLEMENT PATHWAY DEFICIENCIES: Genetic deficiencies have been described. classical and alternative pathway components : c/f: recurrent infection with encapsulated bacteria, particularly Neisseria species Genetic deficiencies of the classical complement pathway (C1, C2 and C4) are associated with a high prevalence of autoimmune disease, particularly severe systemic lupus erythematosus (SLE) Mannose-binding lectin deficiency is very common (5% of the population). Individuals with complete mannose-binding lectin deficiency have an increased incidence of bacterial infections if subjected to an additional cause of immune compromise, such as prematurity or chemotherapy. Deficiency of the regulatory protein C1 inhibitor is not associated with recurrent infections, but causes recurrent angioedema. Investigations: 1. Complement C3 and C4 are the only complement components that are routinely measured. 2. The CH50 (classical haemolytic pathway 50, also known as total haemolytic complement THC) -Complement proteins degrade rapidly at room temperature, and the most common cause of an absent CH50 is delay in transportation of the sample to the laboratory. 3 IMMUNE DEFICENCY Treatment: 1. No definitive treatment of complement deficiencies 2. Patients are at risk of meningococcal and other infections, and should be vaccinated with meningococcal, pneumococcal and H. influenzae B vaccines in order to boost their adaptive immune responses 3. Life-long prophylactic penicillin to prevent meningococcal infection is recommended 4. At-risk family members should be screened for complement deficiencies with functional complement assays. C. PRIMARY DEFICIENCIES OF THE ADAPTIVE IMMUNE SYSTEM 1. COMBINED B- AND T-LYMPHOCYTE IMMUNE DEFICIENCIES Caused by defects in lymphoid precursors Causes recurrent bacterial, fungal and viral infections soon after birth Stem cell transplantation is the only current treatment option, although specific gene therapy is under investigation. 2. PRIMARY T-LYMPHOCYTE DEFICIENCIES Generally present in childhood Characterized by recurrent viral, protozoal and fungal infections Many T-cell deficiencies are associated with defective antibody production( because of the importance of T cells in providing help for B cells) Types: 1) DiGeorge syndrome: Results from failure of development of the 3rd/4th pharyngeal pouch Usually caused by a deletion of 22q11 Associated with abnormalities of the aortic arch, hypocalcaemia, tracheo-oesophageal fistulae, cleft lip and palate, and absent thymic development Characterized by very low numbers of mature T cells despite normal development in the bone marrow. IMMUNE DEFICENCY 4 2) Bare lymphocyte syndromes Caused by absent expression of HLA molecules within the thymus If HLA class I molecules are affected, CD8+ lymphocytes fail to develop, while absent expression of HLA class II molecules affects CD4+ lymphocyte maturation Failure to express HLA class I: is associated with recurrent infections, and systemic vacuities caused by uncontrolled activation of natural killer cells. 3) Autoimmune lymphoproliferative syndrome Caused by failure of apoptosis Characterized by accumulation of lymphocytes and persistence of autoreactive cells Patients develop lymphadenopathy, splenomegaly and a variety of autoimmune diseases IMMUNE DEFICENCY 5 Investigations 1. The principal tests for T-lymphocyte deficiencies are a total lymphocyte count and quantitation of lymphocyte subpopulations by flow cytometry 2. Serum immunoglobulins 3. Functional tests of T-cell activation and proliferation and/or an HIV test Treatment: 1. Anti-Pneumocystis and antifungal prophylaxis 2. Aggressive management of specific infections 3. Immunoglobulin replacement may be indicated if disease is associated with defective antibody production 4. Stem cell transplantation may be appropriate in bare lymphocyte syndromes 5. Thymic transplantation has been used for DiGeorge syndrome 3. PRIMARY ANTIBODY DEFICIENCIES (B lymphocyte deficiency): Primary antibody deficiencies are characterized by recurrent bacterial infections, particularly of the respiratory and gastrointestinal tract The most common causative organisms are bacteria such as S. pneumoniae and H. influenza Severe inherited disorders of antibody production are rare usually present at 5-6 months of age, when the protective benefit of transferred maternal immunoglobulin has waned Three major primary antibody deficiencies present in adulthood: 1) Selective IgA deficiency is the most common primary immune deficiency In most patients, low (< 0.05 g/l) or undetectable IgA is an incidental finding with no clinical sequelae 30% of individuals experience recurrent mild respiratory and gastrointestinal infections. In some patients, there is a compensatory increase in serum IgG levels. 2) Common variable immune deficiency (CVID): Unknown cause Characterised by low serum IgG levels and failure to make antibody responses to exogenous pathogens IMMUNE DEFICENCY 6 Antibody-mediated autoimmune diseases such as idiopathic thrombocytopenic purpura and autoimmune haemolytic anaemia are common Associated with an increased risk of malignancy, particularly lymphoproliferative disease. 3) Specific antibody deficiency or functional IgG antibody deficiency causes defective antibody responses to polysaccharide antigens Some patients are deficient in the antibody subclasses IgG2 and IgG4, and this condition was previously called IgG subclass deficiency 4) There is overlap between specific antibody deficiency, IgA deficiency and CVID, and some patients may progress to a more global antibody deficiency over time IMMUNE DEFICENCY 7 Investigations: 1. Serum immunoglobulins & protein and urine electrophoresis: to exclude secondary causes of hypogammaglobulinaemia 2. Specific antibody :measuring IgG antibodies against tetanus, H. influenzae and S. pneumonia 3. If specific antibody levels are low, immunization with the appropriate killed vaccine should be followed by repeat antibody measurement 6-8 weeks later; failure to mount a response indicates a significant defect in antibody production. 4. Quantitation of B and T lymphocytes by flow cytometry Management 1. Aggressive treatment of infections, and prophylactic antibiotics 2. The mainstay of treatment is immunoglobulin replacement (intravenous immunoglobulin, IVIG), which is derived from pooled plasma and contains IgG antibodies to a wide variety of common organisms. IVIG is usually administered intravenously every 3-4 weeks with the aim of maintaining trough IgG levels within the normal range 3. Treatment may be self-administered, and is life-long. 4. Immunization is generally not effective (because of the defect in IgG antibody production) except in selective IgA deficiency. 5. live vaccines should be avoided IMMUNE DEFICENCY II. SECONDARY IMMUNE DEFICIENCIES 8 IMMUNE DEFICENCY 9 Much more common than primary immune deficiencies Infection is a common cause of secondary immune deficiency, particularly HIV infection, measles and other viral illnesses Immune deficiency is also an expected side-effect of some drugs, particularly those used in the management of transplantation, autoimmunity and cancer May be an idiosyncratic effect of other agents, particularly anti-epileptic medication Physiological immune deficiency occurs at the extremes of life, and the decline of the immune response in the elderly is known as immune senescence IX & Rx the cause if possible.