Ms. Raihan UNIT 1 Mega Quiz Study Guide KEY 1. Draw an atom
advertisement

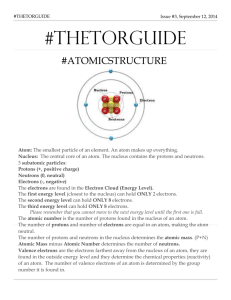
Ms. Raihan UNIT 1 Mega Quiz Study Guide KEY 1. Draw an atom and label its parts. Where is the nucleus located? What is the structure around the nucleus called? Nucleus (protons and neutrons) Electron cloud (electrons) 2. What are the charge and mass of proton, neutron, and an electron? Protons weigh 1 amu and has a +1 charge Electrons weigh 0 amu and has a -1 charge Neutrons weigh 1 amu and has no charge (0) 3. How do you identify an atom on the periodic table? Why? An atom of an element is identified by the number of proton as this remains unchanged. The number of protons is called the atomic number, which is located on top of the element symbol. Number of electrons changes as we have seen for ions and number of neutrons also changes as seen with isotopes. The one particle number that remains unchanged is proton, so it is used to ID an element. 4. If and atoms has 9 protons, 9 electrons and 10 neutrons, what is the identity? How do you know? This atom has 9 protons thus the atomic number is 9. The element with 9 as its atomic number is Fluorine. 5. How do you calculate the # of neutrons in an atom? The number of neutrons can be calculated by subtracting the atomic number from the atomic mass (rounded to a whole number). 6. Compare the mass and the volume of nucleus and the electron cloud. The nucleus consist 99.9% (most of atom’s mass). The nucleus has a large mass but small volume while the electron cloud has a large volume but small (negligible) mass. 7. Why is the nucleus so heavy? Explain. The nucleus is where the protons and neutrons are located. Both of these have a mass of 1 amu respectively making the nucleus the densest part of an atom. 8. Why do atoms tend to gain or lose electrons? All atoms tries to attain stability of the noble gas by completing its octet. The goal of losing or gaining electron is to attain noble gas status by having 8 valence electrons in its outermost energy level. 9. When you gain electrons you become negative while when you lose electrons you become positive. Explain this concept (you may use math or analogies). Electrons each have a -1 charge. As you gain electrons, you are becoming more and more negative as you are gaining subsequent -1 charges. As you lose electrons, you are becoming more and more positive, since the you are losing negative charges. The protons in the nucleus now outnumbers the electrons thus giving you an overall positive charge. Analogies: Any negative things in life will make you more negative (weight, stress, detention hrs.) Taking these negative things away from your life will make you more positive (happy). 10. How can you find the number of valence electrons without drawing Bohr model? The easiest way to determine valence electrons is by looking at the group #. The groups on the periodic table is organized based on valence electrons. For newer periodic table with group #13 to #18, use the last digit (the first 10 electrons will be occupied in the first and second energy levels). 11. Draw the Bohr model and the Lewis Dot notation for Chlorine (#19). What is the difference between these to models? Bohr model shows all the electrons in a particular atom (atomic number). Lewis dot notation only shows the valence electrons( group number). 12. What is the charge of a sulfur (#16) ion? Explain how you found this answer. Sulfur is a nonn-metal and is in group 16. This means it has 6 valence electrons. In order to complete it’s octet Sulfur will gain two electrons giving it a negative charge. Sulfur Ion S2- 13. “Electrons available for bonding” is another term for “Valence Electrons”. Explain what valence electrons are. Valence electrons are found on the outermost energy level of an atom. These are the electrons that are gain, lost, or shared during bonding to fulfill the octet rule. Use the following online review questions to help you prepare for UNIT 1 mega quiz. A score of 80% or higher indicates a proficient level. Type in the URL EXACTLY as shown below. If you can’t open it, you typed it in wrong. Check spelling/Caps etc. Atomic Structure http://www.sciencegeek.net/Chemistry/taters/Unit1AtomicStructure.htm Valence electrons and Dot notations http://www.sciencegeek.net/Chemistry/taters/Unit3ValenceElectrons.htm