Unit 8 Study Guide
advertisement

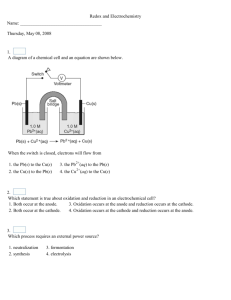
Chemistry – Unit 8 Name:______________________________________ Date:______________________ Per. #:_______ Unit 8 Exam Study Guide NOTE: This is a 30 point homework assignment! Topics Lesson 8.1 – Oxidation and Reduction (pgs. 631-635) o Know all the ways to determine if an element is being oxidized or reduced. o Be able to assign oxidation states to all elements in a chemical reaction. o Be able to use oxidation states to determine if a reaction is redox or not. o Be able to turn word equations into symbolic equations. o Be able to add half reactions to create a balanced overall redox reaction. Lesson 8.2 – Balancing Redox Reactions (pgs. 637-641) o Be able to determine the number of electrons being gained and lost in an unbalanced redox reaction. o Be able to balance a redox reaction using the half reaction method. Memorize the steps! o Remember it is ok and even helpful to leave out spectator ions (ions that do not change oxidation state and therefore are not a part of the redox reaction). Lesson 8.3 – Electrochemical Cells (pgs. 655-657, 662, 665) o Know the parts of an electrochemical cell and be able to describe of even draw a picture of one. o Know the difference between a voltaic (or galvanic) cell and an electrolytic cell. o Know how to identify the anode and the cathode. o Know how electrons would travel in the wire and how ions would migrate across a salt bridge in a cell. o Know the meaning of the standard reduction potential (SRP) and be able to find one for any halfreaction. o Know how to use SRPs to calculate the potential of an oxidation reaction and the overall cell potential (E0cell). o Know how to tell if a redox reaction would be spontaneous or not based on the position of the halfreactions on the SRP handout and the sign of E0cell. o Know how to write the cell notation for any electrochemical cell. Lab 8.1 – Electrolytic Cells (pgs. 667-671) o Know how an overpotential is necessary to drive a nonspontaneous reaction in an electrolytic cell. o Know how to choose between competing possibilities for the oxidation and reduction half reactions to determine which ones would occur in an electrolytic cell. Practice Problems 1. For each of the following, write “R” if the statement describes reduction or “O” if the statement describes oxidation. Occurs at the anode 2 F – F 2 + 2 e– Cu2+ + 2e– Cu° Loses electrons Gains electrons Cl2 + 2 e– 2Cl– Charge on element becomes more negative Electrons on the right side of arrow Occurs at the cathode Charge on element becomes more positive Name:______________________________________ Date:______________________ Per. #:_______ 2. Consider the following half-reactions and E values: Ag+(aq) + e– Ag(s) E = 0.80 V 2+ – Cu (aq) + 2e Cu(s) E = 0.34 V 2+ – Pb (aq) + 2e Pb(s) E = –0.13 V a. Which of these ions is most likely to be reduced? _______ b. Which metal is most likely to be oxidized? _______ Consider this oxidation-reduction reaction: Cu2+ + Pb(s) Pb2+ + Cu(s) Write the reduction half-reaction: Write the oxidation half-reaction: Calculate the voltage created by this redox reaction: (show work) 3. Consider the following half-reactions and E values: Ag+ (aq) + e– Ag(s) E = 0.80 V Cu2+ (aq) + 2e– Cu(s) E = 0.34 V An electrochemical cell is set up. Write the equations for the reactions at each electrode, and the overall reaction. Calculate the value of E°cell. Reaction Cathode: Anode: Overall: E° Name:______________________________________ Date:______________________ Per. #:_______ 4. Standard Reduction Potentials (volts) Ag+(aq) + e– Ag(s) +0.80 I2(s) + 2e– 2I–(aq) +0.535 Cu2+(aq) + 2e– Cu(s) +0.337 2 H+ (aq) + 2 e– H2 (g) Write the equations for the reactions at each electrode, and for the overall equation. Calculate the Cell Voltage. Sn | Zn 0.00 Sn2+(aq) + 2e– Sn(s) –0.14 Cd2+(aq) + 2e– Cd(s) –0.40 Zn2+(aq) + 2e– Zn(s) –0.763 2H2O(l) + 2e– H2(g) + 2OH-(aq) –0.828 Al3+(aq) + 3e– Al(s) Cd | Al –1.66 5. The reactions that occur at each pencil are given. a. Circle the appropriate word(s) from each pair of words. 2 H2O (l) + 2e– H2 (g) + 2 OH– – [ oxidation | reduction ] + [ gains e– | loses e– ] [ anode | cathode ] 2 H2O (l) O2 (g) + 4 H+ + 4 e– [ oxidation | reduction ] [ gains e– | loses e– ] [ anode | cathode ] b. Label the pencil that is connected to the + end of the battery. c. What role does the battery serve? d. If phenolphthalein indicator were added to the liquid in the petri dish, draw in the area that would turn pink. Name:______________________________________ Date:______________________ Per. #:_______ 6. Standard Reduction Potential (volts) Eºreduction O2(g) + 4H+(aq) + 4e– 2H2O(l) Br2(s) + 2e– 2Br–(aq) 2H2O(l) + 2e– H2(g) + 2OH–(aq) Na+(aq) + e– Na(s) Standard Oxidation Potential (volts) +1.23 +1.07 –0.828 –2.71 Eºoxidation Na(s) Na+(aq) + e– H2(g) + 2OH–(aq) 2H2O(l) + 2e– 2Br–(aq) Br2(s) + 2e– 2H2O(l) O2(g) + 4H+(aq) + 4e– +2.71 +0.828 –1.07 –1.23 The pencil apparatus was placed in a solution of NaBr(aq) and universal indicator (acid = red; base = purple) Both Na+ and H2O can be reduced. Circle the half-reaction that will take place. What would you observe? Justify your answer. Both Br– and H2O can be oxidized. Circle the half-reactions that will take place. What would you observe? Justify your answer. 7. In the following reaction: 2 Na (s) + Cl2 (g) 2 Na+ (aq) + 2 Cl– (aq) a. Write the reduction half-reaction. b. Write the oxidation half-reaction. c. How many electrons are transferred during this reaction? ___________ 8. Balance the following redox reactions using the half-rxn method. a. KNO3(s) KNO2(s) + O2(g) b. HNO3(aq) + H2S(g) NO(g) + H2O(l) + S(s) c. SO32–(aq) + MnO4–(aq) SO42–(aq) + Mn2+(aq)