Ch13b - Louisiana Tech University
advertisement

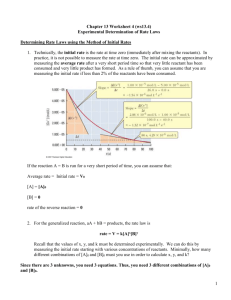
Louisiana Tech University, Chemistry 102 POGIL (Process Oriented Guided Inquiry Learning) Exercise on Chapter 13(2). Chemical Kinetics 2 Why? Chemical kinetics is important because it provides new models, concepts, and parameters to study the rates of chemical reactions. Graphical and method of initial rates have been used to determine the rate law of a chemical reaction. It is important to know different ways of expressing rate laws. The rate law in differential gives the order of reactants, and in the integral form rate constant and concentration of initial and final reactants. The half-life (t½) from is way to easily find how long it will take-to complete a reaction. Most of the chemical reactions follow 1st order rate law; therefore we focus more on first order reactions. All nuclear reactions also follow first order kinetics, i.e. decay of a radioactive material is directly proportional to the number or nuclei of the element. Half-life (t½) from is used more frequently for radioactive decay in place of rate constant (k). Learning Objectives Understand rate laws 3. Determine reaction orders from a rate law, and use the integrated rate and initial rates methods to obtain orders and rate constants (Section 13-3). 4. Calculate concentration from time, time to reach a certain concentration, half-life for a first-order reaction (Section 13-3). Success Criteria Identify rate law for a chemical reaction ( zero, first, or second order) from kinetic data using graphical and initial rate methods. Calculations of reaction time, and final concentration of reactants given initial concentration and vice versa. Resources Chemistry: The Molecular Science 4rd Edition, John W. Moore, Conrad L. Stanitski, Peter C. Jurs -ISBN-10: 1439049300 ISBN-13: 9781439049303 Prerequisites New Concepts Rate Law The rate law is an expression that relates the rate of a chemical reaction to a constant (rate constant) and concentration of reactants raised to a power. The power of a concentration is called the order with respect to the particular reactant. Every chemical reaction has a rate equation based on the rate law. The rate of a chemical reaction is directly proportional to the concentration of reactants raised to a power, which could be an integer: 0, 1, 2, or 3. E.g. a A + b B -----> c C rate [A]l[B]m rate k [A]l[B]m k = rate constant [A] = concentration of A [B] = concentration of B l = order with respect to A ( nothing to do with a, the stoichiometric coefficient) m = order with respect to B First-Order Reactions A first order reaction (order = 1) has a rate proportional to the concentration of one of the reactants. rate = k[A] (or B instead of A), Second-Order Reactions A second-order reaction (order = 2) has a rate proportional to the concentration of the square of a single reactant or the product of the concentration of two reactants: rate = k[A]2 (or rate = k[A] [B]) Mixed-Order or Higher-Order Reactions Mixed-order reactions have a fractional order for their rate: e.g., rate = k[A]1/3 Orders of reactants have to be experimentally determined. There are two way to get it. Graphical method: Reaction: a A b B Order Differential Integrated Rate Law Graph (y) vs. (x) Slope Half-life form t½ Rate Law y =mx + b 0 rate = k 1 rate = k[A] 2 2 [A]t vs. t -k ln[A]t = -kt + ln[A]0 ln[A]t vs. t -k 1/[A]t = kt + 1/[A]0 1/[A]t vs. t k [A]t = -kt + [A]0 rate=k[A] t½ = [A]0/2k t½ = 0.693/k t½ = [A]0/2k For simple reactions we can plot kinetic data. [A] vs. t to get a liner graph For simple reactions we can plot kinetic data E. g. 2 N2O5 ----> 4 NO2 + O2 0.02 0 -2 0 0.015 0.01 [A] 0.005 50 100 -4 0 50 100 time 150 200 200 ln[A] -6 0 150 -8 -10 time [A] vs. t ln [A] vs. t Reaction rate law is: rate = k [N2O5] ; which is first order. Method of Initial Rates: Rate law of more complex reactions involving more than one reactant requires changing concentration of one reactant while holding the other one to see the effect of order on the rate. E.g. For the chemical reaction: A + B ----> C Using the initial rates data given below deduce: [A],mol/L [B],mol/L rate,mol/LS -4 -5 4.6 x 10 3.1 x 10 2.08 x 10-3 -4 -5 4.6 x 10 6.2 x 10 4.16 x 10-3 -4 -5 9.2 x 10 6.2 x 10 1.664 x 10-2 The rate law : rate = k [A]x [B]y a) the order of each reactant Oder of A: 4 = (9.2 x 10-4/(4.6 x 10-4)x = 2x 4 = 2x x = 2 Therefore, the order with respect to A is two or second order. Order of B: The rate law : rate = k [A]x [B]y 2 = (6.2 x 10-5/3.1 x 10-5) y = 2 y 2 = 2y y = 1 Therefore, the order with respect to B is one or first order. Rate law of the reaction: rate = k [A]2 [B] Total order of the reaction 3 or third order. b) the rate constant The rate law for the reaction : A + B ----> C rate = k [A]2 [B]1 plugging the values from one of the experiments: rate = k [A]2 [B]1 _________________________________________________________________________ 2.08 x 10-3 = 2.08 x 10-3 = k k (4.6 x 10-4)x (4.6 x 10-4)2 (3.1 x 10-5)y (3.1 x 10-5)1 _________________________________________________________________________ 2.08 x 10-3 k = ----------------------------- = 3.17 x 108 (4.6 x 10-4)2 x (3.1 x 10-5)1 k = 3.17 x 108 L2/mol2 s The rate constant for the reaction is 3.17 x 108 L2/mol2 s. Calculation using half-life t1/2 form: Half-life is the time required for a reactant to reach half its original concentration ( [A]t = ½ [A]0 First-order reaction is t½ = 0.693/k. The half-life of a first order reaction does not depend on concentration. Integrated rate law: ln[A]t = -kt + ln[A]0; ln(½ [A]0) = -kt + ln[A]0; ln ([A]0/2)= ln [A]0 – ln 2 = -kt + ln[A]0; ln 2 = kt; 0.693 = k t½; t½ = 0.693/k Zero-order rate law is given as t½ = [A]0/2k. Second-order reaction is t½ = 1/(k[A]0). GHW#2 Printed Name:_____________________ Group Name:__________ Chapter 13 (2) Key Questions (relatively simple to answer using the Focus Information) 1. The reaction A ---> B + C is known to follow the rate law: rate = k [A]1 What are the differential, integral and half-life (t½) form of this rate law? 2. Using graphical method, show that 2 N2O5 ---> 4 NO2 + O2, is a first order reaction. [N2O5] / Time / min moldm-3 0 0.01756 20 0.00933 40 0.00531 60 0.00295 80 0.00167 100 0.00094 160 0.00014 3. For the reaction: A ---> D, Find the order of [A] for each case. It was found in separate experiments that a) The rate doubled when [A] doubled b) The rate tripled when [A] tripled c) The rate quadrupled when [A] doubled d) The rate increased 8 times when [A] doubled 4. For the chemical reaction: A + B ----> C Using the following initial data to deduce: a) Order of each reactant b) Rate constant [A],mol/L [B],mol/L rate,mol/Ls _____________________________ 2.0 3.0 0.10 6.0 3.0 0.90 6.0 6.0 0.90 Exercises involving chemical and nuclear reactions 5. The rate constant for the first-order conversion of A to B is 2.22 hr-1. How much time will be required for the concentration of A to reach 75% of its original value? 6. The half-life of a radioactive (follows first order rate law) isotope is 10 days. How many days would be required for the isotope to degrade to one eighth of its original radioactivity? 7. The rate constant for the first order decomposition of SO2Cl2 (SO2Cl2-> SO2 +Cl2) at very high temperature is 1.37 × 10-3 min-1. If the initial concentration is 0.500 M, predict the concentration after five hours (300 min).