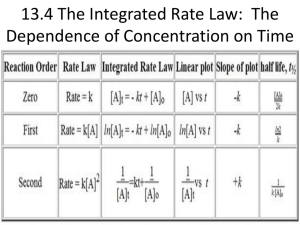
A. Integrated Rate Law of First Order Reactions
advertisement
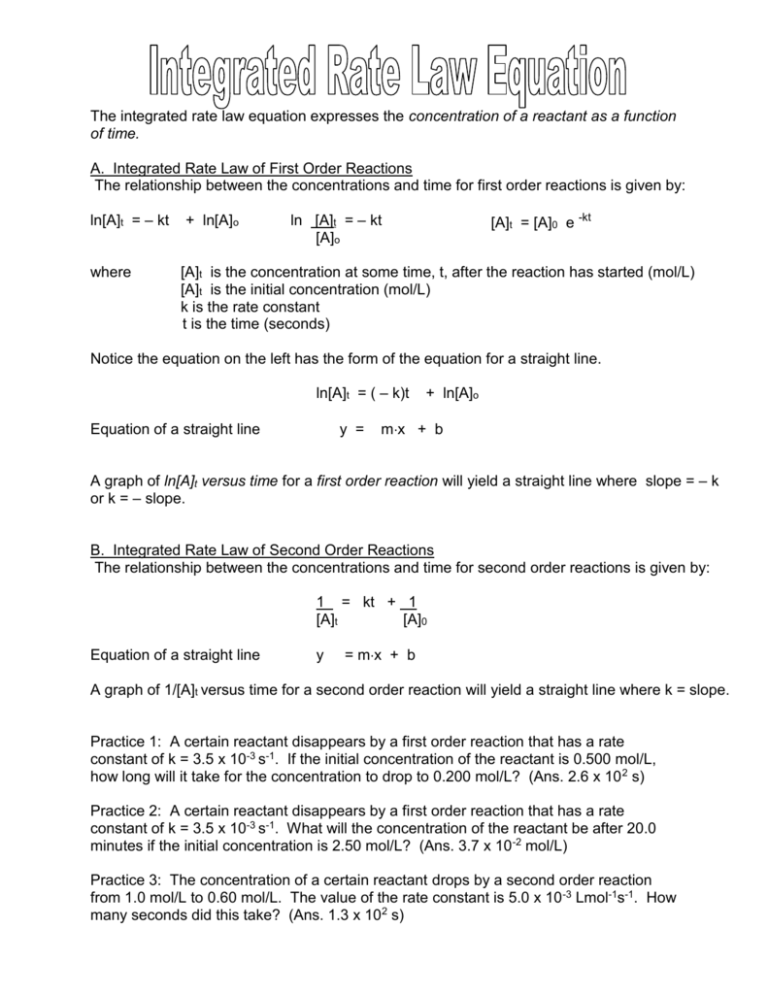
The integrated rate law equation expresses the concentration of a reactant as a function of time. A. Integrated Rate Law of First Order Reactions The relationship between the concentrations and time for first order reactions is given by: ln[A]t = – kt where + ln[A]o ln [A]t = – kt [A]o [A]t = [A]0 e -kt [A]t is the concentration at some time, t, after the reaction has started (mol/L) [A]t is the initial concentration (mol/L) k is the rate constant t is the time (seconds) Notice the equation on the left has the form of the equation for a straight line. ln[A]t = ( – k)t Equation of a straight line y = + ln[A]o mx + b A graph of ln[A]t versus time for a first order reaction will yield a straight line where slope = – k or k = – slope. B. Integrated Rate Law of Second Order Reactions The relationship between the concentrations and time for second order reactions is given by: 1 = kt + 1 [A]t [A]0 Equation of a straight line y = mx + b A graph of 1/[A]t versus time for a second order reaction will yield a straight line where k = slope. Practice 1: A certain reactant disappears by a first order reaction that has a rate constant of k = 3.5 x 10-3 s-1. If the initial concentration of the reactant is 0.500 mol/L, how long will it take for the concentration to drop to 0.200 mol/L? (Ans. 2.6 x 10 2 s) Practice 2: A certain reactant disappears by a first order reaction that has a rate constant of k = 3.5 x 10-3 s-1. What will the concentration of the reactant be after 20.0 minutes if the initial concentration is 2.50 mol/L? (Ans. 3.7 x 10 -2 mol/L) Practice 3: The concentration of a certain reactant drops by a second order reaction from 1.0 mol/L to 0.60 mol/L. The value of the rate constant is 5.0 x 10 -3 Lmol-1s-1. How many seconds did this take? (Ans. 1.3 x 102 s) The half-life, t1/2 , of a reaction is the amount of time required for the concentration of the reactant to decrease to half of its initial concentration time. It is the time during which the amount or its concentration decreases to half of its initial value. That is, at one half-life t = t1/2 at two half-lives t = 2 x t1/2 at three half-lives t = 3 x t1/2 [A]t = ½ [A]0 [A]t = ½ x ½ [A]0 = ¼ [A]0 [A]t = ½ x ½ x ½ [A]0 = 1/8 [A]0 A. Half-Life of First Order Reactions The expression relating half-life of a first order reaction to the value of the rate constant for a reaction is: t1/2 = 0.693 k B. Half-Life of Second Order Reactions The expression relating half-life of a second order reaction to the value of the rate constant for a reaction is: t 1/2 = 1 k[A]0 Practice 1: An isotope has a half-life of 10.6 h. What is the rate constant for this isotope? (Ans. 0.0654h-1) Practice 2: The mass of an antibiotic in a patient is 2.464 g. What mass of antibiotic will remain after 6.0 h, if the half-life is 2.0 h and no further drug is taken? (Ans. 0.31 g)