KHWISERO SUB-COUNTY PRE-M-CATS 2015 CHEMISTRY 233/2
advertisement
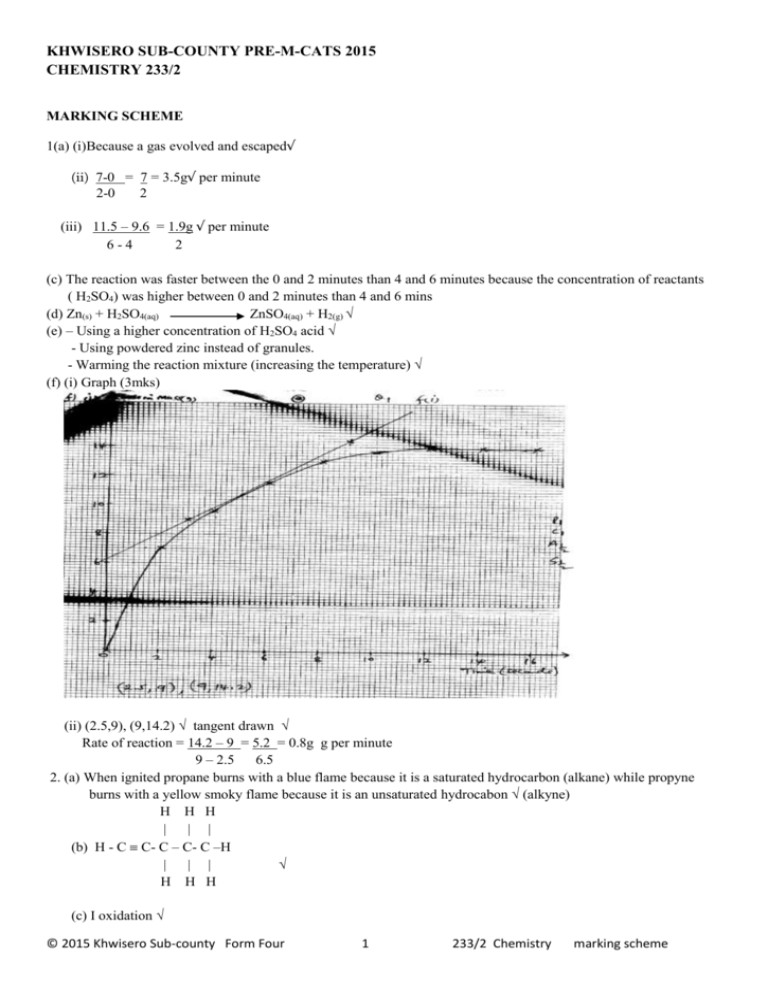
KHWISERO SUB-COUNTY PRE-M-CATS 2015 CHEMISTRY 233/2 MARKING SCHEME 1(a) (i)Because a gas evolved and escaped√ (ii) 7-0 = 7 = 3.5g√ per minute 2-0 2 (iii) 11.5 – 9.6 = 1.9g √ per minute 6-4 2 (c) The reaction was faster between the 0 and 2 minutes than 4 and 6 minutes because the concentration of reactants ( H2SO4) was higher between 0 and 2 minutes than 4 and 6 mins (d) Zn(s) + H2SO4(aq) ZnSO4(aq) + H2(g) √ (e) – Using a higher concentration of H2SO4 acid √ - Using powdered zinc instead of granules. - Warming the reaction mixture (increasing the temperature) √ (f) (i) Graph (3mks) (ii) (2.5,9), (9,14.2) √ tangent drawn √ Rate of reaction = 14.2 – 9 = 5.2 = 0.8g g per minute 9 – 2.5 6.5 2. (a) When ignited propane burns with a blue flame because it is a saturated hydrocarbon (alkane) while propyne burns with a yellow smoky flame because it is an unsaturated hydrocabon √ (alkyne) H H H | | | (b) H - C C- C – C- C –H | | | √ H H H (c) I oxidation √ © 2015 Khwisero Sub-county Form Four 1 233/2 Chemistry marking scheme II R – Propene √ ( Prop – l-ene) C- Sodium propnoate√ III Dehydration√ IV polypropene√ (ii) C3H8 +5O2(g) 3CO2(g) + 4H2O(l) √ (iii) Used to manufacture (i) plastic ropes (ii) plastic crate √ (iii)Plastic chairs 3. (a) Dry HCl gas Conc. H2SO4 acid (b) concentrated sulphuric (vi) acid√ (c) NaCl(s) + H2SO4(l) NaHSO4(aq) + HCl(g) √ (d) (i) Dry HCl gas has no effect on red and blue litmus √ (ii) Dry HCl gas turns blue litmus paper red√ but has no effect on red litmus√ (e) NaCl(s) + H2SO4 (l) NaHSO4(aq) + HCl(g) √ 23 + 35.5 58.5g 22.4 litres 58.5g = 22.4litres of HCl 120 g = 120 X 22.4√ litres of HCl gas 58.5 = 45.95litres √ (f) A white precipitate is formed√ Silver nitrate reacts with hydrogen chloride to form a white precipitate of silver chloride√ 4. (a) (i) H3 = molar heat of combustion of carbon = molar heat of formation of carbon (iv) oxide gas (ii) 2C(s) + 3H2(g) Hf C2H6(g) 7 +02 + 3/2O2 /2O2 2H2 3H3 √ H1 2CO2(g) + 3H2O(l) Hf = 2H2 + 3H3 - H1 =2 (-394) + 3(-286) - ( -1560) √ = -788 + -858 +1560 = - 86kJmole-1 © 2015 Khwisero Sub-county Form Four 2 233/2 Chemistry marking scheme (iii) √½ 2C(s) + 3H2(g) Energy kJ Hf = - 86kJmole-1 √ C2H6(g) √ ½ Reaction path (covese of reaction) (b)(i) Mass x S.H.C X DT = 400 X 4.2 X 19.5 √ = -32760 joules = -32.776 kJ √ (iii) C2H6 = (2 X 12 + 6 X 1) = 30 30g = -1560kJ ? = 32.76kJ -32.76 x 30 √ -1560 = 0.63g√ 5. (a) A solution that contains a maximum amount of solute at a given temperature. √ (ii) I. 38g per 100g of water II. solubility at 40oC = 62/100g of water hence 62g dissolves while 80-62 = 18g remain undissolved © 2015 Khwisero Sub-county Form Four 3 233/2 Chemistry marking scheme (c) At 20oC it has 38g per 100g of water =1000 x 38 100 = 380 g KNO3 per litre KNO3 = 39 + 14+48 = 101 Hence = 380 moles per litre 101 =3.762 molar 3.762MKNO3 6. (a) T(s) + H2O(l) TO(s) + H2(g) (b) an acidic oxide (c) (i) R (ii) A (iii) Y (d) (i)2ANO3(s) 2ANO2(s) + O2(g) (ii) TCO3(s) TO(s) + CO2(g) (e)- A strong acid ionize completely in aqueous solution eg HNO3 HCL,H2SO4 (aqueous) - A weak acid ionize partially in aqueous solution e.g H2CO3 , any organic acid (f) (i) the reaction between an acid and a base to form alt and water only (ii) Place dilute H2SO4 acid in a beaker and add excess copper (II) oxide to ensure that all the acid has reacted. Filter to remove the unreacted(excess) copper (II) oxide Heat the filtrate to concentrate it leave it to cool slowly to form crystals. 7 (a) (i) calcium oxide (ii) the reaction is exothermic (iii) Brine ( or concentrated sodium chloride), ammonia gas and Limestone ( or carbon (iv) oxide gas) (iv) by heating or by thermal decomposition of NaHCO3 (b) (i) CO2 ( or in words) NH3 ( or in words) H2O (b) (ii) Calcium chloride It is sprayed on roads in ice cold countries to melt the ice as an impurity (iii) Fractional crystallization (iv) Because KHCO3 is highly soluble in water hence it will not separate away from NH4Cl at G. © 2015 Khwisero Sub-county Form Four 4 233/2 Chemistry marking scheme