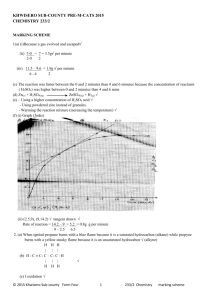
Challenge Problem - nemsgoldeneagles
advertisement
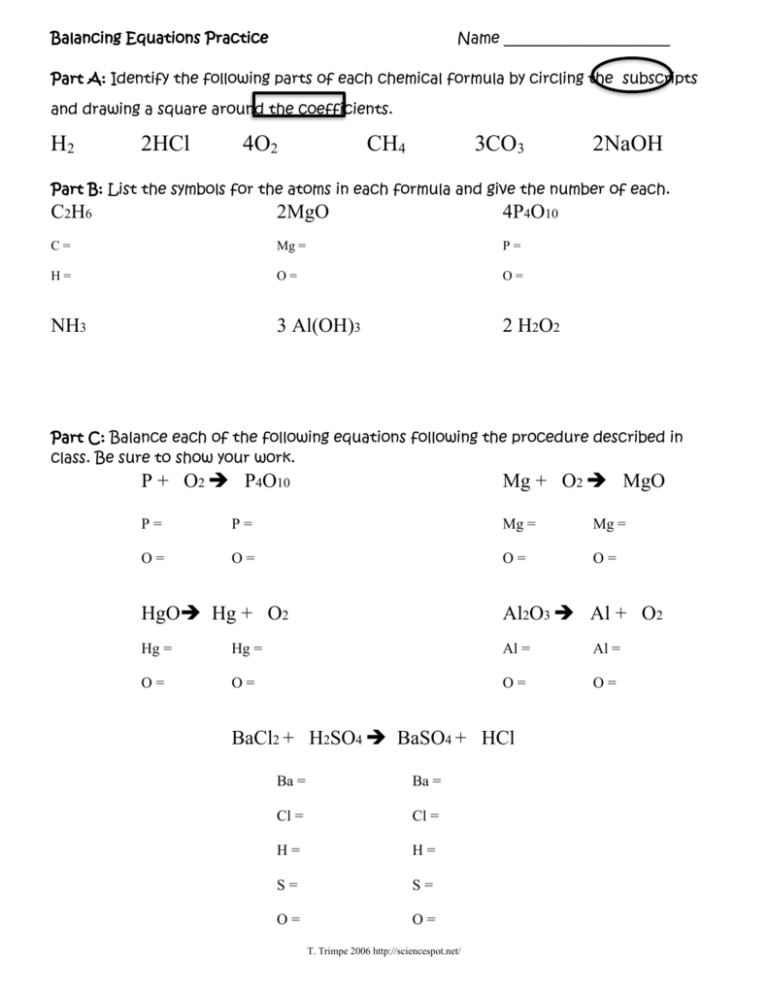
Balancing Equations Practice Name ______________________ Part A: Identify the following parts of each chemical formula by circling the subscripts and drawing a square around the coefficients. H2 2HCl 4O2 CH4 3CO3 2NaOH Part B: List the symbols for the atoms in each formula and give the number of each. C2H6 2MgO 4P4O10 C= Mg = P= H= O= O= NH3 3 Al(OH)3 2 H2O2 Part C: Balance each of the following equations following the procedure described in class. Be sure to show your work. P + O2 P4O10 Mg + O2 MgO P= P= Mg = Mg = O= O= O= O= HgO Hg + O2 Al2O3 Al + O2 Hg = Hg = Al = Al = O= O= O= O= BaCl2 + H2SO4 BaSO4 + HCl Ba = Ba = Cl = Cl = H= H= S= S= O= O= T. Trimpe 2006 http://sciencespot.net/ Part D: Practice Problems – Balance each equation using the process from Part C. Cl2 + NaBr NaCl + Br2 H2 + N2 NH3 Na + Br2 NaBr CuCl2 + H2S CuS + HCl HgO + Cl2 HgCl + O2 C + H2 CH4 Challenge Problem: Give it your best shot! C2H6 + O2 CO2 + H2O T. Trimpe 2006 http://sciencespot.net/ INFORMATION SHEET TO THE WORKSHEET. PART A: H2 HCl O2 CH4 CO3 -Hydrogen as it naturally occurs. -Hydrochloric acid, reacts mostly with metals to -Oxygen as it naturally occurs. -Methane gas; a natural hydrocarbon gas used as a combustible fuel. -“Carbon trioxide can be made by blowing ozone at dry ice (solid CO2), NaOH and it has also been detected in reactions between carbon monoxide (CO) and molecular oxygen (O2).” -Sodium hydroxide a common ingredient in soaps and liquid plumber. PART B: C2H6 MgO P4O10 NH3 Al(OH)3 H2O2 - Ethane; a natural hydrocarbon gas used as a combustible fuel. - Magnesium oxide - Tetraphosphorous decoxide - Ammonia - Aluminum hydroxide - Hydrogen peroxide PART C: P + O2 P4O10 - Phospohorus & oxygen combining to form Tetraphosphorous decoxide will react with water to make phosphoric acid. This is a synthesis reaction. Mg + O2 MgO - The bright white light in most fireworks and flares; Magnesium oxide. This is a synthesis reaction. HgO Hg + O2 - Mercuric oxide is a yellow pigment in paint. When heated the compound decomposes into Mercury metal and oxygen gas. Decomposition reaction. Al2O3 Al + O2 - “By itself aluminum oxide does not decompose. By electrolysis it gets split up into Al and O2.” Decomposition reaction. BaCl2 + H2SO4 BaSO4 + HCl - Barium chloride reacting with sulfuric acid to produce Barium sulfate, which is “used as a … contrast medium in x-ray photography of the digestive tract.” Hydro chloric acid is also formed as a product from this double-displacement reaction. M. W. Hall 2011 PART D: Cl2 + NaBr NaCl + Br2 - Chlorine reacting with Sodium bromide to produce table salt and bromine liquid. Single-displacement reaction. H2 + N2 NH3 - Hydrogen reacts with nitrogen to produce ammonia. This is a synthesis reaction. Na + Br2 NaBr - Sodium reacts with Bromine to produce sodium bromide; the tablets that are placed in hot tubs and pools. This is a synthesis reaction. CuCl2 + H2S CuS + HCl - Copper chloride reacts with Hydrogen sulfide to produce copper sulfide and hydrochloric acid. Decomposition reaction. HgO + Cl2 HgCl + O2 - Mercuric oxide reacts with Chlorine to produce Mercuric chloride and oxygen gas, this is a doubledisplacement reaction. C + H2 CH4 - Carbon reacting with Hydrogen to produce the hydrocarbon methane. This is a synthesis reaction. CHALLENGE PROBLEM: C2H6 + O2 CO2 + H2O - This is a combustion reaction of Ethane burning to produce carbon dioxide and water. M. W. Hall 2011