Section 14.5-14.7
advertisement

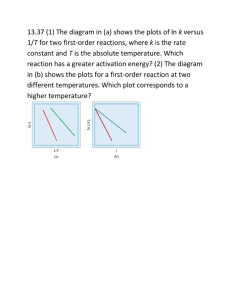
14.5-14.7 1. Define collision model 2. Define orientation factor 3. Define activation energy 4. Define activated complex 5. Define transition state 6. Draw and label a generic energy diagram 7. Define the Arrhenius equation 8. Define frequency factor 9. Consider a series of reactions having these energy profiles: 10. Define equation 14.20 11. The following table shows the rate constants for the rearrangement of methyl isonitrile at various temperatures (these are the data points in Figure 14.14): (a) From these data, calculate the activation energy for the reaction. (b) What is the value of the rate constant at 430.0 K? 12. Define elementary reaction 13. Define molecularity 14. Define unimolecular 15. Define bimolecular 16. Define termolecular 17. Define intermediate 18. Draw a generic energy diagram with at least on transition state 19. It has been proposed that the conversion of ozone into O2 proceeds by a two-step mechanism: O3(g) O2(g) + O(g) O3(g) + O(g) 2 O2(g) (a) Describe the molecularity of each elementary reaction in this mechanism. (b) Write the equation for the overall reaction. (c) Identify the intermediate(s). 20. If a reaction is elementary, its rate law is based directly on its molecularity. Give an example of this statement. 21. If the following reaction occurs in a single elementary reaction, predict its rate law: H2(g) + Br2(g) 2 HBr(g) 22. Define rate determining step. 23. The decomposition of nitrous oxide, N2O, is believed to occur by a two-step mechanism: N2O(g) N2(g) + O(g) (slow) N2O(g) + O(g) N2(g) + O2(g) (fast) (a) Write the equation for the overall reaction. (b) Write the rate law for the overall reaction. 24. Show that the following mechanism for Equation 14.24 also produces a rate law consistent with the experimentally observed one: 25. Define catalysis 26. Define homogenous catalyst 27. Define heterogeneous catalyst 28. Define adsorption 29. Define absorption 30. Define enzyme 31. How do catalytic converters work? 32. Formic acid (HCOOH) decomposes in the gas phase at elevated temperatures as follows: HCOOH(g) CO2(g) + H2(g) The uncatalyzed decomposition reaction is determined to be first order. A graph of the partial pressure of HCOOH versus time for decomposition at 838 K is shown as the red curve in ▶ Figure 14.31. When a small amount of solid ZnO is added to the reaction chamber, the partial pressure of acid versus time varies as shown by the blue curve in Figure 14.31. (a) Estimate the half-life and first-order rate constant for formic acid decomposition. (b) What can you conclude from the effect of added ZnO on the decomposition of formic acid? (c) The progress of the reaction was followed by measuring the partial pressure of formic acid vapor at selected times. Suppose that, instead, we had plotted the concentration of formic acid in units of mol/L. What effect would this have had on the calculated value of k? (d) The pressure of formic acid vapor at the start of the reaction is 3.00 * 102 torr. Assuming constant temperature and ideal-gas behavior, what is the pressure in the system at the end of the reaction? If the volume of the reaction chamber is 436 cm3, how many moles of gas occupy the reaction chamber at the end of the reaction? (e) The standard heat of formation of formic acid vapor is ΔHf° = -378.6 kJ/mol. Calculate ΔH° for the overall reaction. If the activation energy (Ea) for the reaction is 184 kJ/mol, sketch an approximate energy profile for the reaction, and label Ea, ΔH °, and the transition state. 33. (a) What factors determine whether a collision between two molecules will lead to a chemical reaction? (b) According to the collision model, why does temperature affect the value of the rate constant? (c) Does the rate constant for a reaction generally increase or decrease with an increase in reaction temperature? 34. Calculate the fraction of atoms in a sample of argon gas at 400 K that has an energy of 10.0 kJ or greater. 35. The gas-phase reaction Cl(g) + HBr(g) HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction, and label Ea and ΔE. (b) What is the activation energy for the reverse reaction? 36. Indicate whether each statement is true or false. (a) If you compare two reactions with similar collision factors, the one with the larger activation energy will be faster. (b) A reaction that has a small rate constant must have a small frequency factor. (c) Increasing the reaction temperature increases the fraction of successful collisions between reactants. 37. Based on their activation energies and energy changes and assuming that all collision factors are the same, which of the following reactions would be fastest and which would be slowest? (a) Ea = 45 kJ/mol; ΔE = -25 kJ/mol (b) Ea = 35 kJ/mol; ΔE = -10 kJ/mol (c) Ea = 55 kJ/mol; ΔE = 10 kJ/mol 38. (a) A certain first-order reaction has a rate constant of 2.75 * 10-2 s-1 at 20 °C. What is the value of k at 60 °C if Ea = 75.5 kJ/mol ? (b) Another first-order reaction also has a rate constant of 2.75 * 10-2 s-1 at 20 °C What is the value of k at 60 °C if Ea = 125 kJ/mol ? (c) What assumptions do you need to make in order to calculate answers for parts (a) and (b)? 39. The rate of the reaction CH3COOC2H5(aq) + OH- (aq) CH3COO- (aq) + C2H5OH(aq) was measured at several temperatures, and the following data were collected: Calculate the value of activation energy by constructing an appropriate graph. 40. (a) What is meant by the term elementary reaction? (b) What is the difference between a unimolecular and a bimolecular elementary reaction? (c) What is a reaction mechanism? (d) What is meant by the term rate-determining step? 41. What is the molecularity of each of the following elementary reactions? Write the rate law for each. (a) Cl2(g) 2 Cl(g) (b) OCl- (aq) + H2O(l) HOCl(aq) + OH- (aq) (c) NO(g) + Cl2(g) NOCl2(g) 42. (a) Based on the following reaction profile, how many intermediates are formed in the reaction A D? (b) How many transition states are there? (c) Which step is the fastest? (d) For the reaction A D, is ΔE positive, negative, or zero? 43. The following mechanism has been proposed for the gas phase reaction of H2 with ICl: H2(g) + ICl(g) HI(g) + HCl(g) HI(g) + ICl(g) I2(g) + HCl(g) a) Write the balanced equation for the overall reaction. b) Identify any intermediates in the mechanism. c) If the first step is slow and the second one is fast, which rate law do you expect to be observed for the overall reaction? 44. The reaction 2 NO(g) + Cl2(g) 2 NOCl(g) was performed and the following data obtained under conditions of constant (Cl2): (a) Is the following mechanism consistent with the data? NO(g) + Cl2(g) NOCl2(g) (fast) NOCl2(g) + N(g) 2 NOCl(g)(slow) (b) Does the linear plot guarantee that the overall rate law is second order? 45. What is a catalyst? (b) What is the difference between a homogeneous and a heterogeneous catalyst? (c) Do catalysts affect the overall enthalpy change for a reaction, the activation energy, or both? 46. In Figure 14.22, we saw that Br- (aq) catalyzes the decomposition of H2O2(aq) into H2(l) and O2(g). Suppose that some KBr(s) is added to an aqueous solution of hydrogen peroxide. Make a sketch of [Br– (aq)] versus time from the addition of the solid to the end of the reaction. 47. The oxidation of SO2 to SO3 is accelerated by NO2. The reaction proceeds according to: NO2(g) + SO2(g) NO(g) + SO3(g) 2 NO(g) + O2(g) 2 NO2(g) a) Show that, with appropriate coefficients, the two reactions can be summed to give the overall oxidation of SO2 by O2 to give SO3. b) Do we consider NO2 a catalyst or an intermediate in this reaction? c) Is this an example of homogeneous catalysis or heterogeneous catalysis? 48. Many metallic catalysts, particularly the precious-metal ones, are often deposited as very thin films on a substance of high surface area per unit mass, such as alumina(Al2O3) or silica(SiO2). (a) Why is this an effective way of utilizing the catalyst material compared to having powdered metals? (b) How does the surface area affect the rate of reaction? 49. When D2 reacts with ethylene (C2H4) in the presence of a finely divided catalyst, ethane with two deuteriums, CH2D¬CH2D, is formed. (Deuterium, D, is an isotope of hydrogen of mass 2). Very little ethane forms in which two deuteriums are bound to one carbon (for example, CH3¬CHD2). Use the sequence of steps involved in the reaction (Figure 14.24) to explain why this is so. 50. The enzyme carbonic anhydrase catalyzes the reaction CO2(g) + H2O(l) HCO3-(aq) + H+(aq). In water,without the enzyme, the reaction proceeds with a rate constant of 0.039 s-1 at 25 °C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 1.0 * 106 s-1 at 25 °C. Assuming the collision factor is the same for both situations, calculate the difference in activation energies for the uncatalyzed versus enzyme-catalyzed reaction. 51. The activation energy of an uncatalyzed reaction is 95 kJ/mol. The addition of a catalyst lowers the activation energy to 55 kJ/mol. Assuming that the collision factor remains the same, by what factor will the catalyst increase the rate of the reaction at (a) 25 °C, (b) 125 °C?