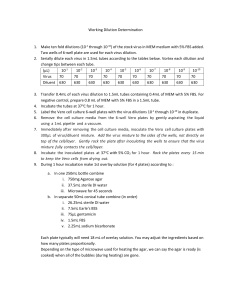
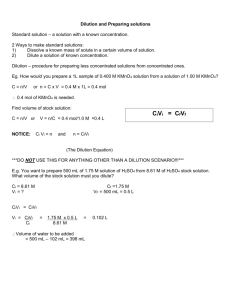
Plaque Reduction Neutralization Test (PRNT) Make 1:5 dilution of

Plaque Reduction Neutralization Test (PRNT)
1.
Make 1:5 dilution of each test serum using 150 µL of serum in 600 µL of MEM 5% FBS media.
2.
Make serial dilutions of 1:10 from 1:5 dilution. Take 250 µL of serum (from 1:5 dilution above) and add it to 250 µL MEM 5% FBS media in second tube.
3.
For negative control, make 1:5 dilution of FBS by mixing 80 µL of FBS in 320 µL of MEM 5% FBS media. Positive control PRNT can also be included in the run (usually the serum for positive control
PRNT is a serum which already known the titer).
4.
Heat inactivate all dilutions in water bath at 56 o C for 30 minutes.
5.
Using serial dilutions, dilute virus according to the Working Dilution Determination result.
Calculate how many working dilutions needed for the reaction. Each dilution of serum samples will need 400 µL of working dilution virus. Do not forget to include positive control PRNT and positive control working dilution.
Dilute the virus using MEM 5% FBS media as diluent.
6.
Place 400 µL of working dilution (from step #5) into a separate tube labeled for each sample to be tested.
7.
Add 400 µL of each sample (after removing from water bath) to the 400 µL of working dilution in its designated tube.
Add 400 µL of PRNT positive control serum into 400 µL of working dilution in designated tube.
(This is the PRNT positive control reaction)
Add 400 µL of MEM 5% FBS media into 400 µL of working dilution in designated tube. (This is the working dilution positive control)
Add 400 µL of MEM 5% FBS media into 400 µL of 1:5 dilution of FBS from step 3. (This is the negative control)
8.
Place serum/virus mixtures into incubator 37 o C with 5% CO
2
for 1 hour.
9.
Remove the cell culture media from the 6well Vero plates by gently aspirating the liquid using a 1mL pipette and a vacuum.
10.
Immediately after removing the cell culture media, inoculate the Vero cell culture plates with
300µL of virus/diluent mixture. Add the virus mixture to the sides of the wells, not directly on top of the cell/layer. Gently rock the plate after inoculating the wells to ensure that the virus mixture fully contacts the cell/layer.
11.
Incubate the inoculated plates at 37 o C with 5% CO
2
for 1 hour . Rock the plates every 15 min to keep the Vero cells from drying out.
12.
During 1 hour incubation make 1st overlay solution (for 5 plates) according to : a.
In one 250mL bottle combine i.
1g Agarose agar ii.
50mL sterile DI water iii.
Microwave for 45 seconds b.
In separate 250mL bottle combine (in order) i.
35mL sterile DI water ii.
10mL Earle’s BSS
iii.
100µL gentamicin iv.
2mL FBS v.
3mL sodium bicarbonate
Each plate typically will need 18 mL of overlay solution. You may adjust the ingredients based on how many plates proportionally.
Depending on the type of microwave used for heating the agar, we can say the agar is ready (is cooked) when all of the bubbles (during heating) are gone.
13.
Underneath a Biosafety cabinet, after 1 hour incubation add 1 st overlay to 6-well plates at 3mL per-well and allow to sit on counter for 10 minutes to allow the agar to solidify, then place in incubator upside down. Make sure the agarose in the 250mL bottle is warm (45 o C – 47 o C) before adding the agarose on the wells. Add the overlay to sides of the wells, not directly on top of the cell/layer . Gently swirl the plate after adding the overlay to incorporate the inoculum into the overlay media.
14.
Incubate the 6-well plates at 37 o C with 5% CO
2 for 3 days.
15.
Prepare the 2 nd overlay according to : a.
Make Neutral Red stock solution in a bottle:
1.
151 mg Neutral Red crystal/powder + 100mL
2.
Autoclave at 115 o C for 10 minutes and store at 4 o C. The neutral red is light
sensitive, cover the bottle with aluminum foil or store it in the dark. b.
Prepare the neutral red solution for 2 nd overlay:
1.
Mix 3 mL neutral red stock solution (from step a above) with 100 mL PBS
(you can also make the solution in 2 batches : 1.5 mL neutral red stock solution (from step a above) + 50 mL PBS, and put the mix of each batch in 50 mL conical tube)
16.
Add 3 mL the neutral red solution (from step b above) into each well of the 6-well plates. Incubate the 6-well plates at 37 o C with 5% CO
2
for 6 hours. Before adding the neutral red solution into the
wells, turn off the biosafety cabinet light since the neutral red is light sensitive.
17.
After the staining is complete, aspirate the neutral red staining solution, ensuring the agarose plug is not disturbed, and then proceed to incubate the plates at 37 o C with 5% CO
2
for overnight.
18.
The wells are then examined for total number of plaques observed. This number is recorded for each dilution tested.