Dilutions and Concentration of Solutions study guide and - Bio-Link

1
Dilutions and Concentration of Solutions study guide and practice problems
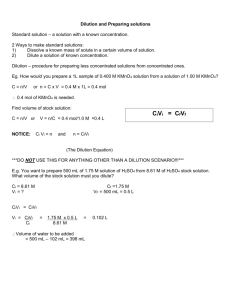
CALCULATING THE CONCENTRATION OF A DILUTED SAMPLE:
You have learned to calculate the dilution of a stock concentration.
However, dilutions are usually referred to by their concentration, in the same way that a stock solution is.
Ex:
Suppose you have made a 1/10 dilution of stock solution A.
Given that stock solution A is at a concentration of 10 mg
NaCl/mL, what is the concentration of the 1/10 dilution of A?
To calculate the concentration of the dilution of A, you simply multiply
the concentration of stock solution A by the dilution:
Dilution = 1/10 X 10 mg NaCl / mL = 1 mg NaCl / mL
CALCULATING THE CONCENTRATION OF A STOCK SOLUTION:
Sometimes, you will know the concentration of the diluted sample, but will want to calculate the concentration of the stock solution used to prepare the dilution:
Ex.
Suppose you have a 1 mg / mL NaCl solution. This solution was prepared by taking 1 mL of Stock Solution A and adding 9 mL of water. What is the concentration of the original Stock Solution A?
To calculate the concentration of Stock Solution A, you first figure out the
dilution:
1 mL stock solution A / 10 mL total = 1/10 dilution
Then, multiply the concentration of the dilution by the INVERSE of the
dilution to calculate the concentration of Stock Solution A:
1 mg / mL NaCl X 10/1 = 10 mg / mL NaCl (for Stock Solution A)
2
Practice problems
1. If you take 2.5 mL of 5 mg/mL glucose and add 7.5 mL of water, what is the concentration of the resulting diluted glucose solution ?
2. If you pipet 10 mL of 100 mM Tris buffer into a graduated cylinder, and bring the total volume of the solution to 150 mL with water, what is the final concentration of the diluted Tris solution ?
3. You have a student helper who made a 20% glycerol solution using the following recipe:
+
30 mL glycerol stock solution
60 mL water
What is the concentration of the student helper used? glycerol stock solution that the
4. You are working in the lab on a test to determine the protein concentration in a given sample (called “A”). You take 50 L of protein sample A and add 950 L of buffer to the sample. You then run your experiment on the diluted protein, and get a concentration of
0.1 mg/mL. What is the concentration of protein in the original protein sample A ?