Radioactivity
advertisement

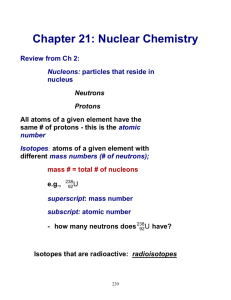
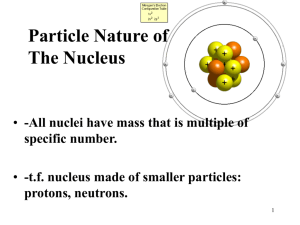
Chemical Vs. Nuclear Reactions 1. Chemical a. ________ are broken or formed. b. Atoms are ____________, but rearranged. c. Involve valence electrons. d. Associated with _________ energy changes. e. Reaction rate influenced by temp, pressure, concentration, catalysts. 2. Nuclear a. __________ emit particles and/or rays. b. Atoms are converted into atoms of another ____________. c. __________ protons, neutrons, and electrons. d. Associated with _______ energy changes. e. Reaction rate not normally affected by temp, pressure, catalysts. Radioactivity ____________ decay – radioactive (unstable) nuclei spontaneously decompose into smaller nuclei Decomposing involves the nuclide (element) emitting a ________ and/or __________ During radioactive decay, atoms of one element are changed into atoms of a different element; a change in ___________ of the element o In order for one element to change into another, the number of _________ in the nucleus must change o All nuclides with ______ or more protons are radioactive ______________ – isotopes of the same atoms with unstable nuclei Undergo radioactive decay to become more _________ Reason? Nuclei are unstable o Gain ____________ by losing _________ We describe nuclear changes with using __________ equations Nuclear Stability Which atomic nuclei are radioactive? Neutrons – add attractive force within the nucleus _________ – related to balance between electrostatic and strong nuclear forces ____________/proton ratio o Low atomic numbers ~1:1 ratio o High atomic numbers ~1.5:1 ratio o Figure 25.8 p. 811 Too ________ neutrons to be stable (above band of stability): o Beta decay, alpha decay, gamma decay Too _______ neutrons to be stable (below band of stability): o Positron emission, electron capture Types of Decay: 1. Alpha Decay – an ∝ particle contains 2 protons and 2 neutrons (helium nucleus) a. loss of an alpha particle means: i. atomic number decreases by 2 ii. mass number decreases by 4 b. Example: 2. Beta Decay – a β particle is like an electron BUT moves much faster, found in the nucleus a. when an atom loses a β particle: i. atomic number increases by 1 ii. mass number remains the same b. in beta decay, a neutron changes into a proton c. Example: 3. Gamma Emission –Gamma ( ) rays are high energy photons a. Occurs when the nucleus rearranges b. No loss of particles from the nucleus c. No change in the composition of the nucleus i. Same atomic number and mass number d. Generally occurs whenever the nucleus undergoes some other type of decay e. Example: 4. Positron Emission – positron has a charge of +1 and negligible mass (anti-electron) a. When an atom loses a positron from the nucleus, its: i. Mass number remains the same ii. Atomic number decreases by 1 b. Positrons appear to result from a proton changing to a neutron c. Example: 5. Electron Capture – occurs when an inner orbital electron is pulled into the nucleus a. no particle emission, but atom changes i. same result as positron emission: b. proton combines with electron to make a neutron i. Mass number remains the same ii. Atomic number decreases by 1 c. Example: Radioactive Decay Rates Radioisotopes have differing decay _________ __________ – time required for one-half of a radioisotope’s nuclei to decay into its products Example: o Strontium-90 half-life (t) = 29 years o Today - 10.0 g strontium o 29 years from now – 5.0 g strontium Equation: amount remaining = initial amount (1/2)n OR amount remaining = initial amount (1/2)t/T t = time elapsed T = duration of one half life *** must have same units of time Table 25.5 p. 818 Radioisotopes all have ____________ half-lives