2014 ChemRepGr5Marked
advertisement

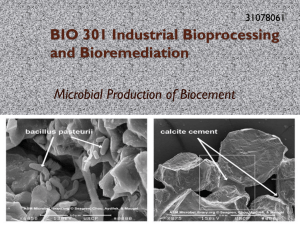
BIO301 Group 5: Chemostat Project Report By Yee Shen Poon (31892966), Ryan McCracken (31864746), Mark Nduru (31609508) and Ong Kiong Teeng (31962909). Abstract Sporosarcina pasteurii was cultured under open (non- sterile) chemostat conditions to produce the enzyme urease. The biomass in the culture was measured using the optical density of the culture solution whereas the amount of urease produced was measured by a conductivity assay which measured the activity of the urease enzyme. Upon increasing the concentration of the substrate from 0.34M urea to 0.68M urea, there was a marked decrease in the biomass of the culture as well as the amount of product, urease, realised. This led the group to the conclusion that 0.68M urea was most likely beyond the optimal substrate concentration for the bacteria resulting in substrate inhibition. Also noted was the consistent high pH of the culture as compared to published material. Overall well written . Results (less biomass with more food supply) seem unusual. Introduction Urease is an enzyme that catalyses the hydrolysis of urea to ammonia and carbonate, which spontaneously decomposes to form carbon dioxide and a second molecule of ammonia. The overall equation for the reaction is: (NH2)2CO + H2O CO2 + 2NH3. Urease is found in numerous fungi, bacteria, algae, plants and some invertebrates. In this experiment, the bacteria Sporosarcina pasteurii (formerly Bacillus pasteurii)was used to produce the urease enzyme. Studies have shown that this bacterium has the ability to precipitate calcite and solidify sand given calcium and a urea source through biological cementation (Siddique & Chahal 2011). The urease produced by these bacteria catalyses the hydrolysis of urea to carbon dioxide and ammonia. The ammonia increases the pH of the surroundings which in turn induces calcite precipitation (Stocks-Fischer et al. 1999). This reaction has applications to improve the durability of certain materials ranging from ornamental stone to soil. The applications of this process to stabilizing embankments, repairing cracked concrete and countering soil liquefaction are of great interest (Bang et al. 2001, Cheng & Cord-Ruwisch 2013, Jonkers & Schlangen 2007, Ramachandran et al. 2001). Well researched and summarised. However, with current biotechnological practices the production of the urease positive bacteria that produce the urease is economically unfeasible due to the cost factor associated with the typical labour and chemical requirements for sterile conditions. The problem is well defined here. Thus, the ability to produce large amounts of urease positive bacteria without the added cost of sterilization would be highly valued. By using selective conditions that favour the growth and survival of urease positive bacteria, it should be feasible to cultivate aforementioned bacteria in non-sterile conditions by creating an environment favourable to the desired bacteria but toxic to undesired bacteria. Urease positive bacteria are alkaliphiles, thriving in high pH environments with high concentrations of free ammonia (NH3). The ammonia comes from the activity of the urease enzyme which aids in maintaining favoured conditions for the alkaliphiles whilst making it harder for non alkaliphile competitors to survive. One such alkaliphile is the bacterium Sporosarcina pasteurii (Achal et al. 2009). The main aim of this experiment was to successfully cultivate S.pasteurii under non-sterile chemostat conditions to produce urease and to observe the effects of varying chemostat conditions on the bacterial biomass and urease production. Excellent Intro Materials and Methods Organism: Sporosarcina pasteurii (supplied by R. Cord-Ruwisch, Murdoch University). Chemostat setup: The Chemostat bioreactor was set up according to the arrangement presented in Flow chart 1. A glass Bioreactor vessel with a working volume of 1L was placed in a 28-30 degrees Celsius water bath with an overhead drill stirrer. An air source was connected to the bioreactor via an airflow tube in order to oxygenate the Chemostat culture. In addition feed and harvest tubes were connected to the bioreactor. The pumps were connected via Labjack card to a computer running Labview software which regulated the inflow of fresh media, the outflow of harvest. Also connected to the Labjack card was a Masterflex micro pump that regulated pH by moving 10M NaOH into the bioreactor if the Labview software detected the pH dropping below the set point of (10.6). Flow chart 1: Arrangement of the Chemostat upon set up: always say where got pictures and graphs from to make sure it is not implied you made it yourself. Chemostat culture inoculation and operation: 670mL of S. pasteurii culture was inoculated into the Chemostat bioreactor at the beginning of the experiment. The culture was continuously fed and harvested for the 7 day duration of the experiment (minus the mandatory shut down of equipment during weekends) at a Hydraulic retention time (HRT) of 16.45 hours with a flow rate of 40.5mL/h. The stirring speed and airflow rate were maintained at a constant 400rpm and 100L/h respectively whilst the water bath was kept within the range of 28-30 degrees Celsius and the pH was monitored by Labview controlled pump to maintain a pH of approximately 10 until the new set point of 9.8 was selected on day 5 (hour 96) of the experiment. Urease activity: Determining Urease activity was achieved through a conductivity assay. The Urease enzyme catalyzes the reaction of Urea into the products of NH4+ and CO32-. The conversion of non-charged reagents to charged products allows for conductivity to be used as a measure of urease activity by measuring the differences in conductivity. The conductivity probe was calibrated in 12.88 units? buffer solution before being inserted into a centrifuge tube filled with 20mL of a solution composed of 10mL (3M) urea, 8mL deionised water and 2mL of the Chemostat culture. The conductivity probe was left for 60 seconds before conductivity readings were taken every minute for 10 minutes. Growth medium (feed): The feed of the Urease positive bacteria (S. pasteurii) was composed of 20g/L Yeast extract, 20.42g/L (0.34M) Urea, 20g/L Sodium acetate and 2mL of 50mM stock/L NiCl2 (0.1mM). The pH of the solution was adjusted to 10 with NaOH after being made up to a volume of 1L with distilled water. After 30 hours the composition of the feed was changed. The concentration of Urea in the feed was changed from 0.34M to 0.68M. After a further 20 hours the feed was again changed; the concentration of urea in the feed was changed from 0.68M to 0.34M. Optical density (Biomass): The Biomass was determined by measuring the optical density using a spectrophotometer. A culture with a higher biomass would have more cells per unit of space hence having a higher population density. As such, when light attempts to pass through the solution, less of it will get through than the same light attempting to pass through a less dense solution due to the reduced amount of cells blocking the path of light. The Optical density was measured by blanking a sample of the feed media and then measuring the absorbance of a sample of the Chemostat culture with the feed as a blank at a wavelength of 600nm. A reading above 2 required diluting the sample and then calculating the OD according to the dilution. pH: The pH was measured using a pH probe. The probe was calibrated before each use according to the provided instructions and the pH adjusted to 10 using 10M NaOH. The labview controlled pump was set tomaintain a pH of approximately 10 until the new set point of 9.8 was selected on day 5 of the experiment. Dissolved Oxygen: The dissolved oxygen content of the Chemostat culture was determined using an oxygen probe. The probe was calibrated according to provided instructions with ppm set as the units of measurement. Results Effect of different urea concentration on urease activity At initial, the feed media contained 0.34M (equivalents with 20.42g/L) of urea under pH of 10.6 for around 30 hours, as shown In Figure 1.After 30 hours, the pH value was controlled in a range of 10.210.3. Meanwhile, the urea concentration in feed media was doubled and halved to test its effect on various aspects: biomass concentration, urease activity and specific urease activity. When the urea concentration was doubled to 0.68M (40.84g/L), the biomass concentration was falling rapidly but then getting back to almost the initial line in a rate. The idea was that you formulate a hypothesis for the test following by accepting or rejecting it from the results obtained. The urease activity kept decreasing all the way, but it stayed at a horizontal level when had come to the valley of biomass concentration. On the other hand, the specific urease activity that initially increased with the dropping of biomass concentration, falling then as the biomass concentration rose exponentially. After 50 hours from set point, the urea concentration was halved to 0.34M. All the three parameters obviously decreased, in particular the biomass concentration and urease activity for 20 hours. At around 70th hour, the urease activity and specific urease activity started to rise sharply until the biomass concentration began to increase, they showed very little growth rate. In final 20 hours, the urease activity stayed virtually constant; while an increase in biomass concentration would lead to a decrease in specific urease concentration. This paragraph is a bit long considering that a paragraph is supposed to make one point. Rather than describing a number of effects, it would be clearer to cover only one effect and then explain the effect (interpretation). In general, an increase in urea concentration positively affects the biomass concentration to grow in a faster rate, where is the evidence for this claim ?) yet decreases the specific urease activity gradually. This might suggest the higher substrate concentration (urea is actually not a true substrate as it does not support growth) in media would stimulate the bacteria population size to increase. (where is the increase?, it seems the interpretation does not link to figure 1) The more dense the bacteria population, specific urease activity will fall (because it is calculated by dividing by the biomass concentration) due to limited factor of substrate concentration.(???) Hence, it may also suggest that the biomass concentration is directly proportional to urease activity, while the biomas(s concentration and specific urease concentration is inversely proportional. (I am wondering whether really your low biomass point at 30 h is a correct value or an outlier. It is not likely that the biomass drops that low in a few hours, if not impossible) Definitely don’t use smooth curve fittings as it often, as also here, is misleading. How culture pH affects the urease production In first 25 hours, a high pH value as 10.6 was applied to chemostat culture media. The Figure 2 (the time plot of figure 2 below looks very similar to that of figure 1. Is that coincidence or actually the same data points?) below illustrates that when in pH 10.6, only the urease activity had obviously increased, the biomass concentration and specific urease activity showed very slow growth rate. Subsequently, when the pH value lowered to 10.2, the biomass concentration decreased sharply for 0.5g/L roughly, but returned to initial level at faster rate. As mentioned above, due to the relationship of biomass amount with specific urease activity, the specific urease activity was improved with the decrease of biomass amount but decreased as the biomass rose to higher level. Could be clearer by using your understanding what specific activity actually means. As the pH 10.2 kept increasing to 10.3 or 10.4, the biomass concentration was no longer increased but falling gradually. The urease activity was dropping as well, however the specific urease activity was not that affected when compared to other two subjects. In fact, the specific urease activity and urease activity started to increase when they reached the turning-point of pH 10.4. All well observed and to be expected. For an excellent report the reader now waits for an explanation that links the findings to your background understanding. From this result, there is significant evidence showing that pH value could manipulate the population of ureolytic bacteria against time. Meanwhile, the farther away the pH value is from 10.0, the bacteria population and urease activity are more likely to decrease. However, when it comes to a certain point, the urease activity and specific urease activity will increase. Discussion The growth of bacteria in chemostat culture was considered as successful experiment. The group was able to recreate the result very similar to our reference. In which way? Back up the claim with numbers. The group also compared its result with other groups to see if there’s any major difference among our data and the finding was that the data was very similar. Good initiative but the results showed no such comparison. Other than just obtained data from 0.34M urea concentration, the urea concentration was changed to 0.68M. The aim of this was to observe if it was possible to increase the yield of the biomass and the product. Nevertheless, changing urea concentration from 0.34M to 0.68M resulted in the biomass concentration falling rapidly but then getting back to almost the initial level in a rate showed in previous results. A possible reason for the drop in biomass concentration after doubling the urea concentration is due to increased toxicity of the biomass living environment, being too toxic for even the S. pasteurii which seem to have the highest activity and biomass at urea concentrations of 0.34M, supported by (Cheng & Cord-Ruwisch 2013) and experiments from group 2's experiment in 2013. This indicates that 0.68M was beyond the optimum urea concentration of S. pasteurii. Using additional changes at different urea concentrations would have allowed more precise showing of the activity of S. pasteurii urease enzyme at different urea concentrations and provided more insight (specifically what insight?) into just how high the concentration of urea can be before it becomes too toxic for S. pasteurii.’ This would have required additional time and further experiments would be recommended to use a longer duration for the experiment with multiple days allowed for measurements to be taken at each of the different urea concentrations. This would be of benefit as the time allows for the bacteria to adjust to the new environment to minimise the effect of potentially recording the lag phase. Had time not been limited, this experiment would have run longer. Although urea is converted into ammonia by the action of urease, the effect of increasing ammonia concentration should not have affected the pH as while ammonia is a base in that it reacts with water to produce a hydroxide ion, it is still a weak (it is not that weak, with a pK value of around 9.5) base as it doesn’t fully convert to a hydroxide ion and the reaction is reversible, rendering the greater majority of ammonia present as ammonia molecules. Comparing the growth and urease activity of the non-sterile chemostat culture to a sterile chemostat culture would have provided a control with which results could be compared to. (True) In (Cheng & Cord-Ruwisch 2013) it was shown that culturing S. pasteurii in sterile conditions is very expensive and in the scope of this experiment, unfeasible for our purposes. In future experiments, if funds allow it, a sterile control culture would allow for a better comparison between the productivity of S. pasteurii in sterile and non sterile culture. Though S. pasteurii has been described by Whiffin (2004) as having similar and sometimes higher urease activity than sterile cultures with contamination as high as 50% (w/w) so it is possible that no significant difference may be observed unless contamination exceeded 50% (w/w). The conditions of growth in the experiment and the use of 40g/L urea feed media showed conditions toxic enough to lyse a significant proportion of the bacterial biomass with the surviving bacteria showing urease activity indicative of S. pasteurii. These conditions would negate high levels of contamination of non extremophiles. With this information the cost of running a sterile culture does not seem justifiable. This experiment has shown that it is possible to culture S. pasteurii under non sterile conditions and as evidenced by (Cheng & Cord-Ruwisch 2013 and Whiffin 2004) the urease productivity is able to be made high enough to pass the 10umol min-1 ml-1 mark required for biocementation. Further lines of research in this area could apply the results and methods learned here for the economically viable production of urease for biocement purposes (as demonstrated by the production of urease for biocementation of silicon sand in group 2's experiment from 2013) as well as in the cultivation of other extremophile bacteria species with valuable products by using conditions selective for them I order to produce aforementioned products in a cost effective manner. The discussion is somewhat less strong than the results. Overall your chemostat has worked quite well as it retained at least 50% of the original pure culture activity. Your chemostat had run at higher pH and higher urea (and hence ammonia) concentration that other chemostats, making conditions quite harsh such that contaminants could not take over (high OD but low urease activity) but instead the B. pasteurii could not grow to its full level at the retention times provided. Overall one of the better reports. 7.5/10 Appendices Hydraulic Retention Time (HRT) and dilution rate calculation: Volume=670mL; flow rate=40.5mL/h HRT=volume/flow rate =670mL/40.5(mL/h) =16.54h D=flow rate/volume =40.5(mL/h)/670mL =0.060h-1 Cheng and Cord-Ruwisch (2013) mentioned that the conductivity values (in mS) can be related to the amount of ammonium produced (in mM) at the end of hydrolysis: 𝑈𝑟𝑒𝑎𝑠𝑒 𝑎𝑐𝑡𝑖𝑣𝑖𝑡𝑦 (𝑚𝑀/𝑚𝑖𝑛) = 𝐶𝑜𝑛𝑑𝑢𝑐𝑡𝑖𝑣𝑖𝑡𝑦 (𝑚𝑆/𝑚𝑖𝑛) ∗ 111.1 =0.125(mS/min)*111.1 =13.888 The specific urease activity of culture can be determined by: 𝑆𝑝𝑒𝑐𝑖𝑓𝑖𝑐 𝑢𝑟𝑒𝑎𝑠𝑒 𝑎𝑐𝑡𝑖𝑣𝑖𝑡𝑦 (𝑚𝑀/𝑚𝑖𝑛/𝑂𝐷) = 𝑈𝑟𝑒𝑎𝑠𝑒 𝑎𝑐𝑡𝑖𝑣𝑖𝑡𝑦 (𝑚𝑀/𝑚𝑖𝑛) 𝑂𝐷 =13.888(mM/min)/3.834 =3.622(mM/min/OD) Meanwhile, the biomass concentration would be determined as: 𝐵𝑖𝑜𝑚𝑎𝑠𝑠 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 (𝑔/𝐿) = 0.44 × 𝑂𝐷(600 𝑛𝑚) =0.44*3.834 =1.687g/L Time (h) pH Conductivity Urease activity (mS/min) (mM/min) [Urea] (g/L) DO OD (mg/L) [Biomass] (g/L) 13.888 Specific urease activity (mM/min/OD) 3.622 0 10.6 0.125 20 4.37 3.834 1.687 27.25 30.25 10.2 10.2 0.148 0.104 16.443 11.554 4.406 6.373 20 40 5.00 5.62 3.732 1.813 1.642 0.798 50.83 10.2 0.090 9.999 2.849 40 2.43 3.510 1.544 74.67 77.98 94.25 99.25 10.3 10.4 10.3 10.3 0.042 0.062 0.067 0.072 4.666 6.888 7.444 7.999 1.942 4.750 4.891 4.522 20 20 20 20 5.25 5.47 5.20 5.46 2.403 1.450 1.522 1.769 1.057 0.638 0.670 0.778 References Achal, V., A. Mukherjee, B.C. Basu and M. Sudhakara Reddy. 2009. “Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production”. Industrial Microbiology.36:pp981–988 Bang, Sookie S., Johnna K. Galinat, and V. Ramakrishnan. 2001. "Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii." Enzyme and Microbial Technology no. 28 (4–5):404409. doi: http://dx.doi.org/10.1016/S0141-0229(00)00348-3. Cheng, L. and R. Cord-Ruwisch. 2013. Selective enrichment and production of highly urease active bacteria by non-sterile (open) chemostat culture. Journal of Industrial Microbiology and Biotechnology, issn 1367-5453 Jonkers, H. M. and E. Schlangen. 2007. “Crack repair by Concrete-Immobilized bacteria”. Proceedings of the First International Conference on Self-Healing Materials.pp1-7 Ramachandran, S. K., V. Ramakrishnan and S.S. Bang. 2001. “Remediation of concrete using microorganisms”. ACI Materials. (98):pp3-9 Siddique, R. and N.K. Chahal. 2011. “Effect of ureolytic bacteria on concrete properties”. Construction and Building Materials. (25)10: pp3791-3801 Stocks-Fischer, Shannon, Johnna K. Galinat, and Sookie S. Bang. 1999. "Microbiological precipitation of CaCO3." Soil Biology and Biochemistry no. 31 (11):1563-1571. doi: http://dx.doi.org/10.1016/S0038-0717(99)00082-6. Whiffin, VS. 2004. "Microbial CaCO3 precipitation for the production of biocement". Ph.D. thesis, Murdoch university, Perth. Western Australia.