Ketones and Aldehydes
advertisement

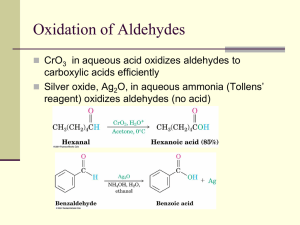
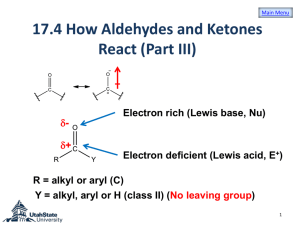
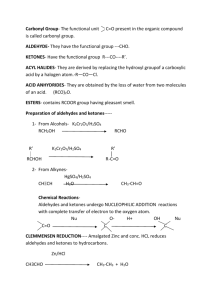
WADE CHAPTER EIGHTEEN OUTLINE Skip: 18-3 through 18-6, 18-8, 18-20B 1) Structure and Physical Properties of Aldehydes and Ketones (brief overview) 2) Synthesis of Aldehydes (review) a) From primary Alcohols with PCC or Swern (DMSO, (COCl)2 b) From terminal alkynes with hydroboration-oxidation (Sia2BH then H2O2, NaOH) c) From cyclic alkenes with reductive cleavage (O3 then Zn or Me2S) 3) Synthesis of Ketones (review) a) From secondary Alcohols with PCC, Swern, or Chromic Acid (H2CrO4) b) From terminal alkynes with oxymercuration – demercuration (HgSO4, H2SO4, H2O) c) Aromatic Ketones via Friedel-Crafts Reaction with benzene (RCOCl, AlCl3) 4) Synthesis of Ketones (new) a) From nitriles with Grignard or organolithium reagents (RCN + R’MgX then H3O+) i) Mechanism (1) Imine Salt Intermediate (2) Hydrolysis of Imine b) From Acid Chlorides with Organocuprates (R’COCl + R2CuLi) 5) Synthesis of Aldehydes (new: Section 18-10) a) From Esters with relatively insoluble hydride reagent DIBAL-H then H3O+ b) From Acid Chlorides with relatively unreactive hydride reagent LiALH(OtBu)3 6) Reactions of Ketones and Aldehydes 7) Nucleophilic Addition of Water a) (acid or base catalyzed mechanism: Mechanism 18-3) b) reversible 8) Nuclophilic Addition of alcohols a) Rxn with alcohols to form Hemiacetals and Acetals (Mechanism 18-6) b) reversible c) Acetal Protecting group with ethylene glycol (Section 18-18) 9) Nucleophilic Addition of Amines to form Imines (Mechanism 18-5) a) Buffered catalysis b) Hydroxylamines, hydrazines and semicarbazide variations (Summary, p 854) 10)Nucleophilic Addition of hydride (NaBH4 or LAH); Review 11)Nucleophilic Addition of carbon a) Acetylide ion (review) b) Cyanide ion (Mechanism 18-4) c) Grignard/ Organolithium (review) d) The Wittig reaction (Section 18-12) i) Formation of the phosphorus ylide PhP=CHR (Ph3P + 1°RX followed by BuLi) ii) Reaction of the ylide with ketone or aldehyde to form alkene via fourmembered cyclic oxaphosphetane intermediate 12)Reduction of C=O to CH2 a) Clemmenson Zn(Hg)/ HCl (Section 17-11B) b) Wolff-Kishner NH2NH2, KOH i) Formation of Imine ii) Hydrolysis of Imine to lose N2 gas (Mechanism 18-7) 13)Selective Oxidation of Aldehydes: Tollens reagent (Ag(I) in ammonia) Homework: 18-37abegh, 40, 42, 44, 46, 47, 48, 50cbd, 51, 57, 59, 60, 64c, 65, 66, 70