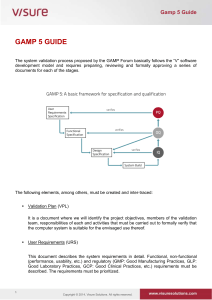
Independent Solutions Tel:(087) 8240529/ (087) 8179688 www
advertisement

Independent Solutions Tel:(087) 8240529/ (087) 8179688 www: www.independentsolutions.ie JOB REFERENCE: INDEP08 Independent Solutions is a professional Irish based limited company with a strong team of full time employees. Our employees come from within the Pharmaceutical Industry with extensive experience. Independent Solutions delivers impartial advice, practical solutions and methodologies to the Pharmaceutical, Biotechnology, Medical Devices and Healthcare Industries. Areas of Expertise: o Project Management (PM) o Lean Validation/Qualification (Process, System, Cleaning & Equipment) o Information Services (IS) /Technology o Regulatory Compliance (EU, FDA and IMB standards) o Quality System Implementation/Review (Many ISO standards) o Guidelines (GMP, GAMP) Clients include: Wyeth, Pfizer (Newbridge, Dunlaoighre), GlaxoSmithKline (Dungarvan), Almac Clinical Services (Craigavon), Gilead (Cork & Dublin), Gerard Laboratories (Mylan) (Dublin), Time and Data Security (TDS) Citywest, Dublin, Mainstream Renewable Energy (Sandyford, Dublin) Shire Medical (Citywest, Dublin), Vistakon (Limerick),National Pathology laboratories, REFERENCE: INDEP08 – Senior Equipment Validation Engineer Job Description: Supporting the activities of Operations / Engineering / Information Management in assuring compliance with the pertinent regulations. Conduct validation activities in compliance with US and EU regulations, global procedures and EHS requirements. Work with the various departments / groups in development and execution of validation activities associated with new equipment / product or System upgrades. Provide input into all project phases i.e. from design through to the commissioning and qualification execution phases of the project. Prioritize qualification activities in line with the project schedules and business needs. Develop Validation Documentation to support new process/product introductions and existing business continuity and process improvement requirements Review and approve documents prepared by the validation team, other departments and contractor organisations (e.g. commissioning test plans, impact assessments, change controls). Execute qualification protocols as required including risk assessments and regulatory reviews Resolve and assist in the closure of deviations initiated during qualification execution. Complete 21 CFR Part 11 and Annex 11 Assessments Implementation and co-ordination of the change control process, review change controls for validation impact , promotes timely approval of all supporting documentation. Generate SOP’s / other documentation as applicable. Validation representative at daily project management meetings, weekly change control and team meetings. Work with all departments in ensuring operational effectiveness and developing product Quality for business continuity and process / product introductions. Proven track record in development / execution of Validation programs in areas of Equipment, Process ,Information management & cleaning Run / Manage complaint investigation and resolution of same. All employees are responsible for minimising both the Environmental and Health & Safety effects of the work that they perform. Experience & Requirements Relevant 3rd Level Qualification in science or engineering Minimum of 4-5 years direct experience in a validation role in either the Medical Device or Pharmaceutical industry. Strong knowledge of CSV/GAMP, 21CFR Part 11, Annex 11, Project Life Cycle and cGMP Regulations, Firm sense of accountability, ownership for end-to-end project lifecycle and sound knowledge of project management. Good knowledge of statistical techniques in the use of problem solving / data analysis. Outstanding verbal and written communication and presenting skills with the ability to interact with technical and non-technical groups. Adaptable and flexible Ability to demonstrate standards of leadership - managing complexity / Credo values / Innovations / Customer Focus Flexible Team Player Good influencing skills