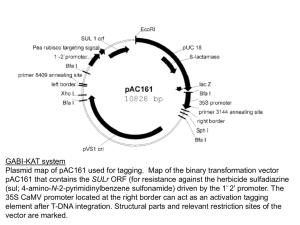
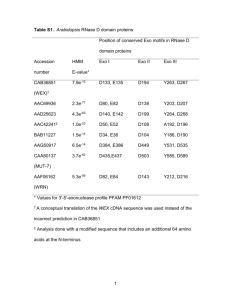
tpj13055-sup-0019-Legends
advertisement

Supporting Information Figure S1. Phylogeny of Bsister genes.A RAxML tree was calculated for all known monocot and representative eudicot Bsister genes as well as the Amborellatrichocarpahomologswith bootstrap values given on the nodes. GGM13 from Gnetumgnemonserved as representative of theoutgroup. OsMADS29-, OsMADS30- and OsMADS31-subclades are indicated by purple, blue and green branches, respectively.Gene phylogeny within subclades follows species phylogeny with two exceptions: The ortholog of OsMADS30 from Phyllostachys is basal within the OsMADS30 subclade in our phylogeny but would be expected to be closely related to the OsMADS30-like genes from Oryza species (Kellogg 2001, 1998). Also, the OsMADS30 ortholog of Hordeum vulgare would be expected to be the sister gene to the subclade of OsMADS30-like genes of Triticum and Aegilops species, but instead it appears to be closely related to one OsMADS30-like gene from Triticumurartu. The branch leading to the OsMADS29 subclade and the branches within this subclade are short, indicating only few substitutions in the OsMADS29-like genes, whereas the branch leading to the OsMADS31 subclade is much longer, which implies considerable sequence changes before species divergence. Figure S2. Alignment of Bsister proteins from grasses (Poaceae), Amborella and Gnetum. Protein sequences of OsMADS29-, OsMADS30- and OsMADS31-like proteins as well as Bsister protein sequences from Gnetumgenom and Amborellatrichopodawere aligned. MADS, I, K and C-terminal domain are designated by bars above the alignment. The heterologous Cterminal domain of OsMADS30 encoded by the OsME is highlighted in red. PI-derived and Paleo-AP3 motif are indicated by boxes. The alignment is colored according to the ClustalX coloring scheme. The mean pairwise identity within the MADS domain of OsMADS29-like proteins and OsMADS31-like proteins is 97 % and 93 %, respectively, while for OsMADS30- like proteins it is only 73 %. Furthermore, almost all OsMADS30-like proteins lack the PI derived motif and have a distorted Paleo AP3 motif. Figure S3. Transmembrane prediction for OsMADS30.“DAS”(Dense Alignment Surface)profile score of OsMADS30 predicted on the basis of its amino acid sequence. Protein domain structure is depicted on top. Calculated at http://www.sbc.su.se/~miklos/DAS/ Figure S4. Alignment of the OsMADS30 genomic locus.The nucleotide sequence of the genomic locus of OsMADS30(chr_OsM30) was aligned to the OsMADS30 mRNA (mRNA_OsM30), OsME1, OsME2, OsME3, the putative ancestral C-terminal region (ancstrC) and published mRNA transcribed from OsME2 (J090038P19) and OsME3 (001033-F05; J013110N09). Figure S5. Sequence features of OsME. (a) Results from a RepBase search using OsME1 as a query. Searching RepBase (Jurka, et al. 2005), we found that a region of 298 bp of the OsME shows a similarity of about 70 % to a non-coding region of an En/Spm transposable element and a 40 bp region with similarity to a MuDR transposable element. (b, c) A dot plot comparison of OsME1 to itself was conducted with seaview (Gouy et al. 2010). For LTRs and TIRs diagonal lines would be expected in the corners of the diagram. Window size and identical sites per window were 4/4 (b) and 11/20 (c). Figure S6. OsMADS30asRNA and in situ hybridization probes. OsMADS30 mRNA is schematically drawn on top. The encoded domains are given above. Coding regions are filled black and blue, UTRs filled white. Regions encoded by OsME are bordered orange. The exons are indicated by broken grey lines. Red, brown and orange boxes below the OsMADS30mRNA indicate cloned or PCR amplified fragments of complementary asRNA and probes used for in situ hybridization, respectively. Figure S7. Expression of Bsister genes in grasses. Data obtained from genevestigator, qTeller and ESTs. White, grey and black boxes indicate no, weak and medium/strong expression, respectively. See Supplementary Table 1 for details and references. nd, no data. Figure S8. OsMADS30 cDNA of T-DNA lines.(a)cDNA from rice plants with and without TDNA insertion was used to amplify PCR products. These were used as a template for nested PCRs with two different primer combinations (A, B) located within OsMADS30 (forward primer, upstream T-DNA insertion) and within the GFP gene (reverse primer, part of T-DNA). The PCR product was verified through sequencing. (b) RNA extracted from 7 day old seedlings with and without T-DNA insertion was used for cDNA synthesis. Primer pairs were located both upstream (A), and up and downstream (B) of the T-DNA insertion of osmads302. Actin1 was used as control.+ , plasmid DNA with the respective cloned cDNA. Figure S9. Mature carpels of Oryza sativa. Toluidine stained mature carpels of theOryza sativa japonicawild type (a) and the T-DNA insertion lines osmads30-1 (b) and osmads30-2 (c). Scale bars = 200 µm. a, antipods; cw, carpel wall; es, embryo sac; n, nucellus. Figure S10. Seeds of OsMADS30 T-DNA insertion lines.Seed set (a), length (b), width (c) and weight (d) of T-DNA insertion lines and wildtype control plants. * and ** indicate sibling plants obtained from a selfed heterozygous plant.Differences between nontransformedDonjing cv. plants and mutant lines did occur. However, they were not correlated with the mutant allele (osmads30-1 and osmads30-2) but rather with the T-DNA insertion line itself, as they were found in wildtype siblings of the respective mutant line as well. Hence we do not consider the mutation ofOsMADS30 to be the cause of the variation of respective traits. Figure S11. Grain germination of Oryza sativa OsMADS30T-DNA insertion lines and control plants. The fraction of germinated grains was determined for osmads30-1 (a) and osmads30-2 (b) under different concentrations of NaCl (0 mM (duplicated), 171 mM, 207 mM) and PEG 6000 (3%) daily over the course of ten days. Significant differences between the germination rate of the Donjing cv. and both osmads30-1 and osmads30-2grains could not be detected. * and ** indicate that seeds were taken from sibling plants. The differences in germination rate of osmads30-1* and Dongjin* under high salt conditions (a) is likely not correlated with OsMADS30 since the effect cannot be seen in the second TDNA line. Figure S12. Growth rate (a), compactness (b) and plant height (c) of OsMADS30 T-DNA insertion lines and wild type Oryza sativa.Boxplots for each day of imaging (117-147 days) of wild type control Oryza sativa plants of the cultivar Dongjin (white background) and osmads30-1 (light grey background) and osmads30-2 (dark grey background). (a) Growth rates did not significantly differ between wild type and mutant plants. (b, c) osmads30-1 plants were found to have a significantly lower plant height (although being comparable in terms of projected leaf area) while being significantly more compact than osmads30-2 and wild type plants which could be due to different plant morphological features such as differences in tiller or leaf number (see also Table S3, S4). Probably due to the lower plant height (with shorter leaves/tillers) the hull area of osmads30-1 is furthermore significantly smaller than that of osmads30-2 at almost all observed days (Supplementary Table 3). Since theprojected shoot area (side) is only weakly different between the two mutants (significant only at days 145 and 147), the calculated compactness value (the fraction of the convex hull area filled with plant pixels) of osmads30-1 is significantly higher than that of osmads30-2. This feature distinguishes osmads30-1 also clearly (and significantly) from the wild type. Table S1. Expression data of Bsistergenes in grasses. Table S2. Genes used for phylogeny Table S3. Extracted values of selected phenotypic traits Table S4: Imaging derived phenotypic traits of 3 rice genotypes for day 117 and day 147 after sowing. Table S5. Primer and Probes for quantitative RT-PCR. Methods S1.Supplementary Experimental Procedures.