COGSTATE Study - Phoenix Children`s Hospital
advertisement

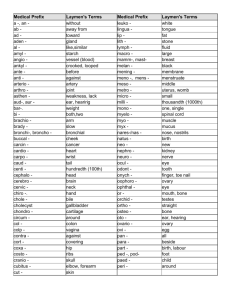
Phoenix Children’s Hospital Research Institute (PCRI) Website Clinical Trial Listing ** Please complete and return to Shy Walker at swalker@phoenixchildrens.com Study Title: Changes in Neurocognition as a Marker of Progressive Disease in Pediatric Brain Tumors Study Purpose (2-3 sentences in laymen terms): The aims of this study are to determine if changes in thinking, reasoning, and problem-solving happen before changes are noted on a routine MRI scan in children and young adults who have received therapy for a brain tumor and to determine if these changes can be detected with testing performed at home. Study Summary (1 paragraph in laymen terms): After the completion of therapy, the brain tumor will be monitored by regular MRI scans to see if it returns. This study includes the use of at-home as well as in-clinic neurocognitive computer testing. Additionally, a quality of life questionnaire will be administered. Basic Eligibility Criteria (1 paragraph in laymen terms): Children and adolescents 6-21 years of age who are fluent in English and have been treated and are stable or have no evidence of disease are eligible for this study if they had one of the following tumors: Ependymoma Glioma (WHO grades I, II, III, and IV) Atypical teratoid rhabdoid tumor (ATRT) Choroid plexus carcinoma Germ cell Medulloblastoma Supratentoral primitive neuroectodermal (sPNET) Study Location(s): Phoenix Children’s Hospital Study Contact(s): Dr. Amy Rosenfeld, Principal Investigator ARosenfeld@PhoenixChildrens.com 602.933.0916 602.933.0920 (Clinic)