eDoC Workgroup & Additional Attachment Templates IG for HL7
advertisement
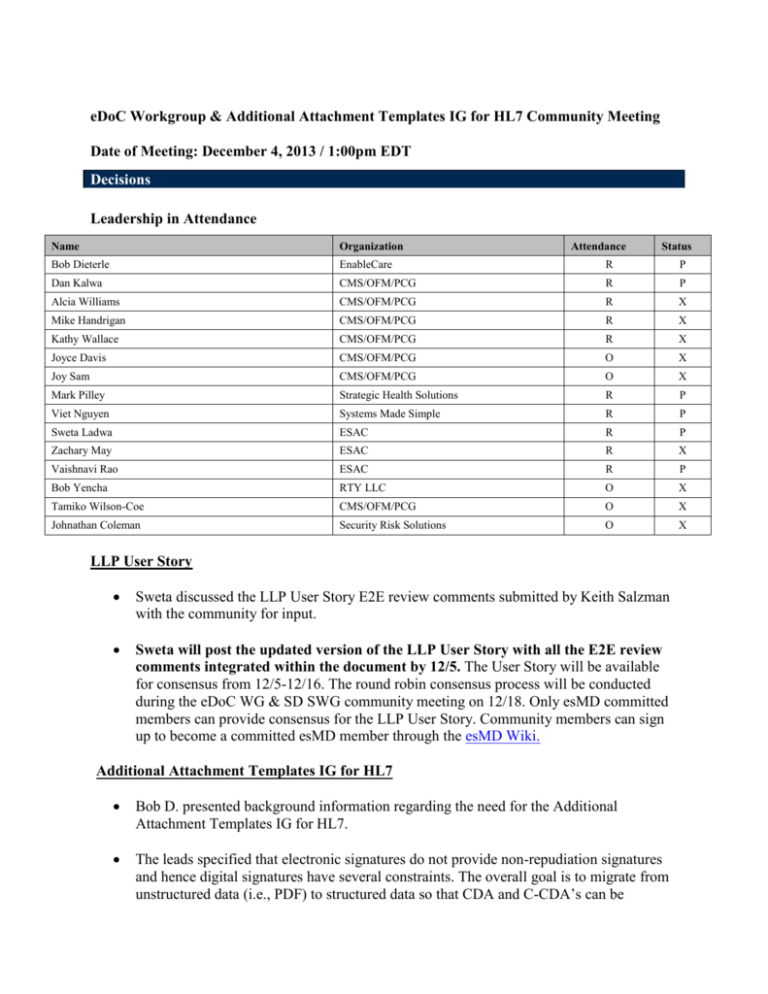
eDoC Workgroup & Additional Attachment Templates IG for HL7 Community Meeting Date of Meeting: December 4, 2013 / 1:00pm EDT Decisions Leadership in Attendance Name Organization Attendance Status Bob Dieterle EnableCare R Dan Kalwa CMS/OFM/PCG R P Alcia Williams CMS/OFM/PCG R X Mike Handrigan CMS/OFM/PCG R X Kathy Wallace CMS/OFM/PCG R X Joyce Davis CMS/OFM/PCG O X Joy Sam CMS/OFM/PCG O X Mark Pilley Strategic Health Solutions R P Viet Nguyen Systems Made Simple R P Sweta Ladwa ESAC R P Zachary May ESAC R X Vaishnavi Rao ESAC R P Bob Yencha RTY LLC O X Tamiko Wilson-Coe CMS/OFM/PCG O X Johnathan Coleman Security Risk Solutions O X LLP User Story Sweta discussed the LLP User Story E2E review comments submitted by Keith Salzman with the community for input. Sweta will post the updated version of the LLP User Story with all the E2E review comments integrated within the document by 12/5. The User Story will be available for consensus from 12/5-12/16. The round robin consensus process will be conducted during the eDoC WG & SD SWG community meeting on 12/18. Only esMD committed members can provide consensus for the LLP User Story. Community members can sign up to become a committed esMD member through the esMD Wiki. Additional Attachment Templates IG for HL7 Bob D. presented background information regarding the need for the Additional Attachment Templates IG for HL7. The leads specified that electronic signatures do not provide non-repudiation signatures and hence digital signatures have several constraints. The overall goal is to migrate from unstructured data (i.e., PDF) to structured data so that CDA and C-CDA’s can be P produced. We want to create an environment in which an individual can refer to a third party for validating a signature within a clinical document. When a clinical document is signed and its contents have been attested to, the signature that is a part of the document will exist on the document until the document no longer has validity. In the current CDA document structure, the CDA header contains a signature text element where all the artifacts of a digital signature and its delegation of rights are stored. When a CDA is signed, a digest is created and the digest creates a more concise and consolidated version of the clinical document. The CDA signature and validation process serves multiple purposes: 1) To ensure that a received document is signed and sent without any alterations 2) To ensure that the sender has signed it and only the receiver’s public key can decrypt the signed digest 3) To ensure the authenticity of the signer. The benefit of using a digital signature process is using a non-repudiation signature which will allow for the verification and integrity of a signed clinical document. We are trying to provide an electronic vehicle that functions within the CDA by providing homes for structured information through document types. The AAT C-CDA R2 sections and entries spreadsheet outlines 5 new document types which include every section that applies to each document type (for a single F2F encounter). There are 4 new constrained sections that can contain additional documents that are not already specified within the C-CDA R2. The 4 new defined sections are: 1) Additional Documentation Section 2) Externally Defined CDE Section 3) Orders Placed Section 4) Transportation Section. Action Items Name Sweta Subject Task Post the updated version of the LLP User Story LLP User Story with all the E2E review comments integrated within the document, Due Date 12/4